Instructions HMEpC
Cell Apps Flyer Epithelial Cells
MSDS Cryopreserved Cells
5 Important Cell Culture Rules
Cell Apps Poster Primary Cells
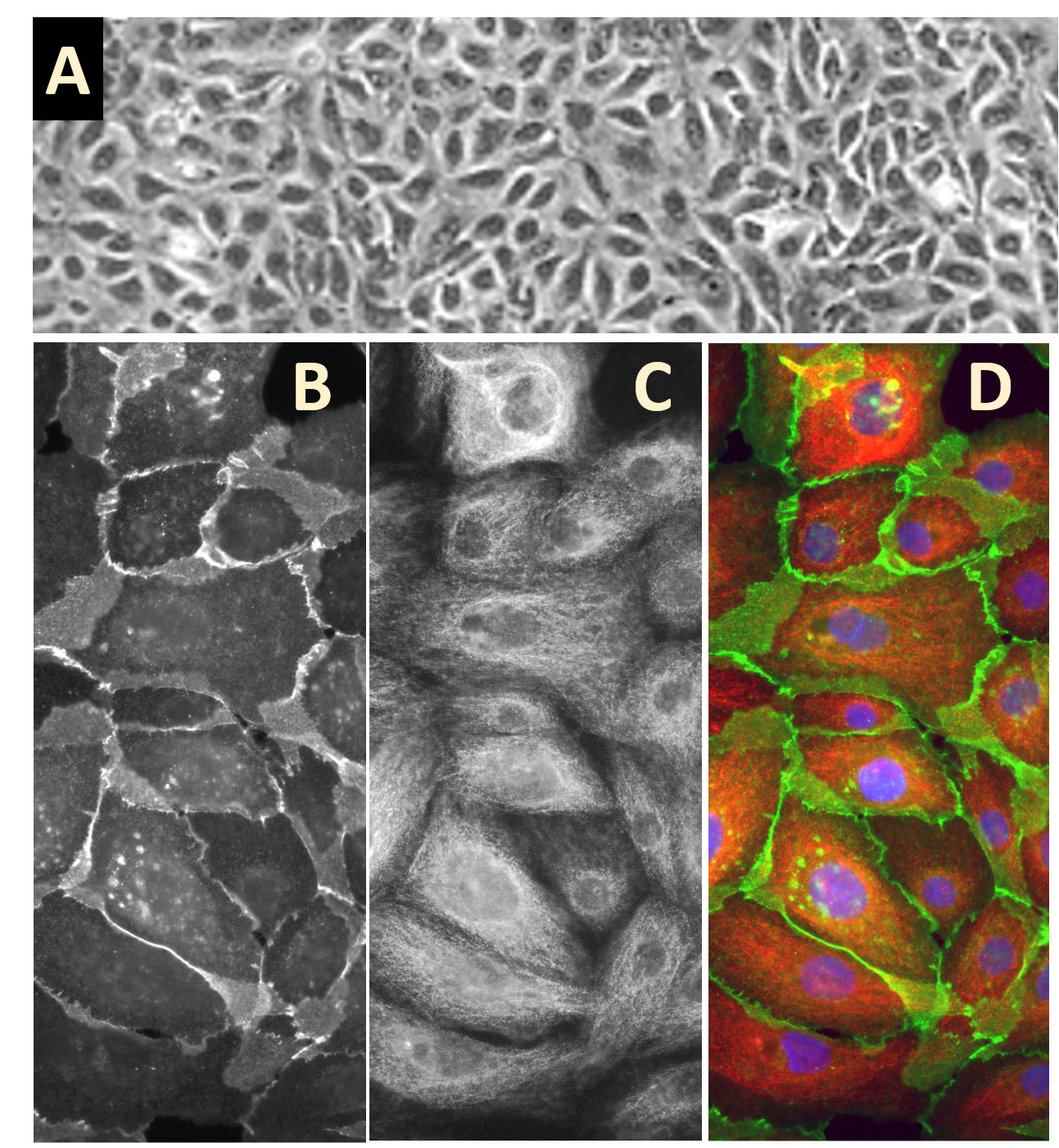
Description
Human Mammary Epithelial Cells (HMEpC) provide an excellent model system to study many aspects of epithelial function and disease, particularly those related to cancerogenesis.
HMEpC from Cell Applications, Inc. have been utilized in numerous research publications, for example to:
- Investigate the role of exosomes secreted by cancer cells in formation of tumor permissive microenvironment through manipulation of normal mammary epithelium
- Serve as control in a study investigating antitumor properties of cannabinoids and stem cell microenvironment
- Determine that differential expression of glycoproteins allows classification of human breast cells into normal, benign, malignant, basal, and luminal groups
- Identify ALDH isoform 5A1 as a potential target for treatment of human breast ductal carcinoma
- Determine that combination of an anti-growth factor treatment using phototherapy (UV-B) is more effective
- Investigate cell survival following DNA damage
- Show the important roles of tumor suppressors in mammary epithelial differentiation
- Explain protection of extracellular matrix from degradation in normal mammary epithelia,
- Show that Maspin loss in metastatic cancer leads to unrestricted ECM degradation, contributing to metastasis, and that loss of EcSOD expression also promotes invasiveness by disrupting ECM
- Investigate the role of shortened telomeres in initiation of genomic instability, cytokinesis failure and polyploidy
- Elucidate the role of Myc in malignancy by studying its ability to transform primary epithelial cells
- Demonstrate, along with Human dermal Fibroblasts, also from Cell Applications, Inc., that resveratrol inhibits mono-ubiquitination of histone H2B
Characterization: Morphology consistent with epithelial origin, and positive for epithelial cell marker cytokeratin 18
Details
Tissue | Normal healthy human mammary glands |
QC | No bacteria, yeast, fungi, mycoplasma, virus |
Bioassay | Attach, spread, proliferate in Growth med |
Cryovial | 500,000 HMEpC (5th passage) frozen in Basal Medium w/ 10% FBS, 10% DMSO |
Kit | Cryovial frozen HMEpC(830-05a), Growth Med (815-500), Subculture Rgnt Kit (090K) |
Proliferating | Shipped in Gr Med, 6th psg (flasks or plates) |
Doublings: | At least 16 |
Applications | Laboratory research use only (RUO). Not for human, clinical, diagnostic or veterinary use. |
Products
| Product | Size | CAT.# | Price | Quantity |
|---|---|---|---|---|
| Cryopreserved Human Mammary Epithelial Cell Total Kit, adult: 5x10^5 Cells (Adult), Medium & Subculture Reagents (See Details tab for specifics) | Size: 1 Kit | CAT.#: 830K-05a | Price: $941.00 | |
| Cryopreserved Mammary Epithelial Cells (HMEpC), adult: Frozen HMEpC (5x10^5) | Size: 1 Cryovial | CAT.#: 830-05a | Price: $746.00 | |
| Proliferating Mammary Epithelial Cells (HMEpC), adult: Actively growing, dividing cells in medium | Size: T-25 Flask | CAT.#: 831-25a | Price: $746.00 | |
| Proliferating Mammary Epithelial Cells (HMEpC), adult: Actively growing, dividing cells in medium | Size: T-75 Flask | CAT.#: 831-75a | Price: $936.00 | |
| Proliferating Mammary Epithelial Cells (HMEpC), adult: Actively growing, dividing cells in medium | Size: 24 Well | CAT.#: 831-24Wa | Price: $936.00 | |
| Proliferating Mammary Epithelial Cells (HMEpC), adult: Actively growing, dividing cells in medium | Size: 96 Well | CAT.#: 831-96Wa | Price: $1,056.00 |
Related Products
| Product | Size | CAT.# | Price | Quantity |
|---|---|---|---|---|
| HMEpC Growth Medium: All-in-one ready-to-use | Size: 500 ml | CAT.#: 815-500 | Price: $136.00 | |
| HMEpC Growth Medium Kit: Basal medium & growth supplement sold together packaged separately | Size: Yields 500 ml | CAT.#: 815K-500 | Price: $148.00 | |
| HMEpC Basal Medium: Basal medium (contains no growth supplement). Add GS before use. | Size: 500 ml | CAT.#: 814-500 | Price: $88.00 | |
| HMEpC Growth Supplement: Added to Basal Medium to create Growth Medium | Size: 5 ml | CAT.#: 815-GS | Price: $87.00 |
Extended Family Products
| Product | Size | CAT.# | Price | Quantity |
|---|---|---|---|---|
| Subculture Reagent Kit: 100 ml each of HBSS, Trypsin/EDTA & Trypsin Neutralizing Solution | Size: 1 Kit | CAT.#: 090K | Price: $69.00 | |
| Mammary Epithelial Cell RNA (HMEpC RNA), Adult: Total RNA prepared from Human Mammary Epithelial Cells, adult | Size: 10 ug | CAT.#: 830-R10a | Price: $398.00 | |
| Mammary Epithelial Cell RNA (HMEpC RNA), Adult: Total RNA prepared from Human Mammary Epithelial Cells, adult | Size: 25 ug | CAT.#: 830-R25a | Price: $796.00 | |
| Monoclonal IL-1 Receptor Antagonist Antibody: Monoclonal IL-1 Receptor Antagonist Antibody | Size: 100 ul | CAT.#: CB19846 | Price: $302.00 | |
| Freezing Medium: For general cryopreservation of most primary cells. Contains FBS & DMSO. | Size: 50 ml | CAT.#: 040-50 | Price: $54.00 | |
| Cytofect Epithelial Cell Transfection Kit (124 x 24-Wells): 124 x 24-Well Rxns | Size: 1 Kit | CAT.#: TF102K | Price: $512.00 | |
| Cytofect Epithelial Cell Transfection Sample Kit (25 x 24-Wells): 25 x 24-Well Rxns | Size: 1 Sample Kit | CAT.#: TF102KS | Price: $68.00 | |
| Human Epidermal Growth Factor (EGF): Human Epidermal Growth Factor | Size: 100 ug | CAT.#: RP1026-100 | Price: $86.00 | |
| Human Epidermal Growth Factor (EGF): Human Epidermal Growth Factor | Size: 500 ug | CAT.#: RP1026-500 | Price: $194.00 | |
| Human Epidermal Growth Factor (EGF): Human Epidermal Growth Factor | Size: 1000 ug | CAT.#: RP1026-1000 | Price: $264.00 | |
| Human IL-1 alpha ELISA Kit: Human Interleukin-1 alpha ELISA Kit | Size: 96 wells | CAT.#: CL0389 | Price: $500.00 | |
| Human Interleukin-1-Alpha (IL-1 alpha): Human Interleukin-1-alpha | Size: 10 ug | CAT.#: RP1173-10 | Price: $194.00 | |
| Human Interleukin-1-Alpha (IL-1 alpha): Human Interleukin-1-alpha | Size: 100 ug | CAT.#: RP1173-100 | Price: $624.00 | |
| Human Interleukin-1-Alpha (IL-1 alpha): Human Interleukin-1-alpha | Size: 1000 ug | CAT.#: RP1173-1000 | Price: $5,327.00 | |
| Human IL-1 beta ELISA Kit: Human Interleukin-1 beta ELISA Kit | Size: 96 wells | CAT.#: CL0392 | Price: $587.00 | |
| Human EGF, Animal-Free: Human Epidermal Growth Factor, Animal-Free | Size: 100 ug | CAT.#: RP1026AF-100 | Price: $95.00 | |
| Human EGF, Animal-Free: Human Epidermal Growth Factor, Animal-Free | Size: 500 ug | CAT.#: RP1026AF-500 | Price: $213.00 | |
| Human EGF, Animal-Free: Human Epidermal Growth Factor, Animal-Free | Size: 1000 ug | CAT.#: RP1026AF-1000 | Price: $290.00 |
Resources/Documents
Citations
Publications
2016
Fite, K., L. Elkhadragy, and J. Gomez-Cambronero. 2016. A Repertoire of MicroRNAs Regulates Cancer Cell Starvation by Targeting Phospholipase D in a Feedback Loop That Operates Maximally in Cancer Cells. Mol Cell Biol, 36:1078-1089.
Pamarthy, S., L. Mao, G. Katara, S. Fleetwood, A. Kulshreshta, A. Gilman-Sachs and K. Beaman. 2016. The V-ATPase a2 isoform controls mammary gland development through Notch and TGF-β signaling. Cell Death & Dis, 7:e2443.
Pampalona, J., E. Roscioli, W. Silkworth, B. Bowden, A. Genesca, L. Tussell and D. Cimini. 2016. Chromosome Bridges Maintain Kinetochore-Microtubule Attachment throughout Mitosis and Rarely Break during Anaphase. PLoS ONE 11(1):e0147420.
Armstrong, M., M. Stang, Y. Liu, J. Yan, E. Pizzoferrato, & J. Yim. 2015. IRF-1 inhibits NF-κB activity, suppresses TRAF2 and cIAP1 and induces breast cancer cell specific growth inhibition. Cancer Biology & Therapy,DOI:10.1080/15384047.2015.1046646.
Komatsu, N., Y. Fujita, M. Matsuda, and K. Aoki. 2015. mTORC1 upregulation via ERK-dependent gene expression change confers intrinsic resistance to MEK inhibitors in oncogenic KRas-mutant cancer cells. Oncogene , 23 February | doi:10.1038/onc.2015.16
2014
Khalkhali-Ellis, Z., W. Goossens, N. Margaryan, and M. Hendrix. 2014. Cleavage of Histone 3 by Cathepsin D in the involuting mammary gland. PLoS ONE, July 23.
Dutta, S., C. Warshall, C. Bandyopadhyay, D. Dutta and B., Chandran. 2014. Interactions between Exosomes from Breast Cancer Cells and Primary Mammary Epithelial Cells Leads to Generation of Reactive Oxygen Species Which Induce DNA Damage Response, Stabilization of p53 and Autophagy in Epithelial Cells. PLoS ONE, 9:e97580.
Teoh-Fitzgerald M, M. Fitzgerald, W. Zhong, R. Askeland, and F. Domann. 2014. Epigenetic reprogramming governs EcSOD expression during human mammary epithelial cell differentiation, tumorigenesis and metastasis. Oncogene, 33:358-368.
2013
Holtzhausen, A., C. Golzio, T. How, Y.-H. Lee, W.P. Schiemann, N. Katsanis, and G.C. Blobe. 2013. Novel bone morphogenetic protein signaling through Smad2 and Smad3 to regulate cancer progression and development. The FASEB Journal. 28:1-20.
Sarkar, S., S. Rajput, A.K. Tripathi, and M. Mandal. 2013. Targeted therapy against EGFR and VEGFR using ZD6474 enhances the therapeutic potential of UV-B phototherapy in breast cancer cells. Molecular cancer. 12:122.
Teoh-Fitzgerald, M., M. Fitzgerald, W. Zhong, R. Askeland, and F. Domann. 2013. Epigenetic reprogramming governs EcSOD expression during human mammary epithelial cell differentiation, tumorigenesis and metastasis. Oncogene.doi: 10.1038/onc.2012.582.
Timpe, L.C., R. Yen, N.V. Haste, C. Litsakos-Cheung, T.-Y. Yen, and B.A. Macher. 2013. Systemic alteration of cell-surface and secreted glycoprotein expression in malignant breast cancer cell lines. Glycobiology. 23:1240-1249.
Yen, T.-Y., S. Dutta, C. Litsakos-Cheung, A. Corona, L. Timpe, and B. Macher. 2013. Overcoming Challenges and Opening New Opportunities in Glycoproteomics. Biomolecules. 3:270-286.
2012
Hendrix, M., L. Postovit, R. Seftor, and E. Seftor. 2012. Methods of inhibiting tumor cell aggressiveness using the microenvironment of human embryonic stem cells. Patent US 8106004 B2.
Kaur, H., S. Mao, Q. Li, M. Sameni, S.A. Krawetz, B.F. Sloane, and R.R. Mattingly. 2012. RNA-Seq of Human Breast Ductal Carcinoma In Situ Models Reveals Aldehyde Dehydrogenase Isoform 5A1 as a Novel Potential Target. PloS one. 7:e50249.
Pampalona, J., C. Frías, A. Genescà, and L. Tusell. 2012. Progressive telomere dysfunction causes cytokinesis failure and leads to the accumulation of polyploid cells. PLoS genetics. 8:e1002679.
Teoh-Fitzgerald, M.L.T., M.P. Fitzgerald, T.J. Jensen, B.W. Futscher, and F.E. Domann. 2012. Genetic and Epigenetic Inactivation of Extracellular Superoxide Dismutase Promotes an Invasive Phenotype in Human Lung Cancer by Disrupting ECM Homeostasis. Molecular Cancer Research. 10:40-51.
2011
Gao, Z., M.S. Xu, T.L. Barnett, and C.W. Xu. 2011. Resveratrol induces cellular senescence with attenuated mono-ubiquitination of histone H2B in glioma cells. Biochemical and biophysical research communications. 407:271-276.
Hendrix, M., L. Postovit, R. Seftor, and E. Seftor. 2011. Methods of inhibiting tumor cell aggressiveness using the microenvironment of human embryonic stem cells. Patent Application US 20130102541 A1.
Yen, T.-Y., B.A. Macher, C.A. McDonald, C. Alleyne-Chin, and L.C. Timpe. 2011. Glycoprotein Profiles of Human Breast Cells Demonstrate a Clear Clustering of Normal/Benign versus Malignant Cell Lines and Basal versus Luminal Cell Lines. Journal of proteome research. 11:656-667.
2010
Hendrix, M., L. Postovit, R. Seftor, and E. Seftor. 2010. Methods of inhibiting tumor cell aggressiveness using the microenvironment of human embryonic stem cells. Patent Application US 20100273707 A1.
Lloyd, S. 2010. An Analysis of Estrogen Metabolism and Breast Cancer Risk. PhD Dissertation, U Pittsburgh.
Windhorst, S., R. Fliegert, C. Blechner, K. Möllmann, Z. Hosseini, T. Günther, M. Eiben, L. Chang, H. Lin, W. Fanick, U. Schumacher, B. Brandt and G. Mayr. 2010. Inositol 1,4,5-Trisphosphate 3-Kinase-A Is a New Cell Motility-promoting Protein That Increases the Metastatic Potential of Tumor Cells by Two Functional Activities. J Biol Chem, 285:5541-5554.
2009
Soler, D., J. Pampalona, L. Tusell, and A. Genescà. 2009. Radiation sensitivity increases with proliferation-associated telomere dysfunction in nontransformed human epithelial cells. Aging Cell. 8:414-425.
Thibodeaux, C., X. Liu, G. Disbrow, Y. Zhang, J. Rone, B. Haddad, and R. Schlegel. 2009. Immortalization and transformation of human mammary epithelial cells by a tumor-derived Myc mutant. Breast Cancer Res Treat. 116:281-294.
2008
Bailey, C.M., D.E. Abbott, N.V. Margaryan, Z. Khalkhali-Ellis, and M.J.C. Hendrix. 2008. Interferon Regulatory Factor 6 Promotes Cell Cycle Arrest and Is Regulated by the Proteasome in a Cell Cycle-Dependent Manner. Mol. Cell. Biol. 28:2235-2243.
Khalkhali-Ellis, Z., D.E. Abbott, C.M. Bailey, W. Goossens, N.V. Margaryan, S.L. Gluck, M. Reuveni, and M.J.C. Hendrix. 2008. IFN-γ regulation of vacuolar pH, cathepsin D processing and autophagy in mammary epithelial cells. J. Cell. Biochem. 105:208-218.
Postovit, L.-M., N.V. Margaryan, E.A. Seftor, D.A. Kirschmann, A. Lipavsky, W.W. Wheaton, D.E. Abbott, R.E.B. Seftor, and M.J.C. Hendrix. 2008. Human embryonic stem cell microenvironment suppresses the tumorigenic phenotype of aggressive cancer cells. Proceedings of the National Academy of Sciences. 105:4329-4334.
Tusell, L., D. Soler, M. Agostini, J. Pampalona, and A. Genescà. 2008. The number of dysfunctional telomeres in a cell: one amplifies; more than one translocate. Cytogenetic and Genome Research. 122:315-325.
2007
Khalkhali-Ellis, Z., and M.J.C. Hendrix. 2007. Elucidating the Function of Secreted Maspin: Inhibiting Cathepsin D–Mediated Matrix Degradation. Cancer research. 67:3535-3539.
Wozniak, R., W. Klimecki, S. Lau, Y. Feinstein, and B. Futscher. 2007. 5-Aza-2'-deoxycytidine-mediated reductions in G9A histone methyltransferase and histone H3 K9 di-methylation levels are linked to tumor suppressor gene reactivation. Oncogene, 26:77-90.
2006
Ligresti, A., A.S. Moriello, K. Starowicz, I. Matias, S. Pisanti, L. De Petrocellis, C. Laezza, G. Portella, M. Bifulco, and V. Di Marzo. 2006. Antitumor activity of plant cannabinoids with emphasis on the effect of cannabidiol on human breast carcinoma. The Journal of pharmacology and experimental therapeutics. 318:1375-1387.
Solomon, J.M., R. Pasupuleti, L. Xu, T. McDonagh, R. Curtis, P.S. DiStefano, and L.J. Huber. 2006. Inhibition of SIRT1 Catalytic Activity Increases p53 Acetylation but Does Not Alter Cell Survival following DNA Damage. Mol. Cell. Biol. 26:28-38.