MSDS Cryopreserved Cells
Instructions BAOSMC
Cell Apps Flyer Smooth Muscle Cells
Cell Apps Flyer Cardiovascular Cells
5 Important Cell Culture Rules
Cell Apps Poster Primary Cells
Cell Applications Inc Brochure
Description
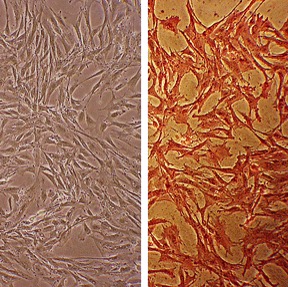
Bovine Aortic Smooth Muscle Cells (BAOSMC) provide an excellent model system to study all aspects of cardiovascular function and disease, especially those related to mechanisms of hyperplasia and hypertrophy of intimal smooth muscle cells leading to vascular occlusion in atherosclerosis and stent restenosis.
BAOSMC from Cell Applications, Inc. have been utilized in a number of research studies, for example, to:
- Elucidate RhoA-dependent serum response signaling pathways
- Observe the role of FAK in thrombospondin-1 induced migration of vascular smooth muscle cells
- Demonstrate the relationship between the disturbed flow and proliferation of smooth muscle cells, leading to intima hyperplasia
- Study interactions between lipoproteins and extracellular matrix, as well as mechanisms of lipoprotein aggregation contributing to atherosclerosis
- Construct an expression cassette to maximize targeted transgene expression
- Test new technologies, such as on-chip contact imaging technique, handheld fluorometers and a “nose on a chip” olfactory sensor
- Study the behavior of smooth muscle cells in 3d environment and smooth muscle/endothelial co-cultures
- Describe an origami-like method of generating three-dimensional (3D) cell-laden microstructures
- Design materials for tissue engineering and cardiovascular implants
Characterization: positive for smooth muscle cell specific alpha-actin expression
Details
Tissue | Normal healthy bovine aorta |
QC | No bacteria, yeast, fungi, mycoplasma |
Character | Smooth muscle specific α-actin positive |
Bioassay | Attach, spread, proliferate in Growth Med |
Cryovial | 500,000 BAOSMC (2nd passage) frozen in Basal Medium w/ 10% FBS, 10% DMSO |
Kit | Cryovial frozen BAOSMC(B354-05), Growth Medium (311-500), Subcltr Rgnt Kit (090K) |
Proliferating | Shipped in Gr Med, 3rd psg (flasks or plates) |
Doublings | At least 16 |
Applications | Laboratory research use only (RUO). Not for human, clinical, diagnostic or veterinary use. |
Products
| Product | Size | CAT.# | Price | Quantity |
|---|---|---|---|---|
| Cryopreserved Bovine Aortic Smooth Muscle Cell Total Kit: 5x10^5 Cells, Medium & Subculture Reagents (See Details tab for specifics) | Size: 1 Kit | CAT.#: B354K-05 | Price: $889.00 | |
| Cryopreserved Bovine Aortic Smooth Muscle Cells (BAOSMC): Frozen BAOSMC (5x10^5) | Size: 1 Cryovial | CAT.#: B354-05 | Price: $715.00 | |
| Proliferating Bovine Aortic Smooth Muscle Cells (BAOSMC): Actively growing, dividing cells in medium | Size: T-25 Flask | CAT.#: B355-25 | Price: $715.00 | |
| Proliferating Bovine Aortic Smooth Muscle Cells (BAOSMC): Actively growing, dividing cells in medium | Size: T-75 Flask | CAT.#: B355-75 | Price: $905.00 | |
| Proliferating BAOSMC: Actively growing, dividing cells in medium | Size: 24 Well | CAT.#: 355-24W | Price: $665.00 | |
| Proliferating Bovine Aortic Smooth Muscle Cells (BAOSMC): Actively growing, dividing cells in medium | Size: 96 Well | CAT.#: B355-96W | Price: $1,025.00 |
Related Products
| Product | Size | CAT.# | Price | Quantity |
|---|---|---|---|---|
| Bovine SMC Basal Medium: Basal medium (contains no growth supplement). Add GS before use. | Size: 500 ml | CAT.#: B310-500 | Price: $74.00 | |
| Bovine SMC Growth Medium: All-in-one ready-to-use | Size: 500 ml | CAT.#: B311-500 | Price: $121.00 | |
| Bovine SMC Growth Medium Kit: Basal medium & growth supplement sold together packaged separately | Size: Yields 500 ml | CAT.#: B311K-500 | Price: $130.00 | |
| Bovine SMC Induction Medium: Provides signals that change cell behavior | Size: 250 ml | CAT.#: B311I-250 | Price: $71.00 | |
| Bovine SMC Growth Supplement: Added to Basal Medium to create Growth Medium | Size: 50 ml (2 parts) | CAT.#: B311-GS | Price: $74.00 |
Extended Family Products
| Product | Size | CAT.# | Price | Quantity |
|---|---|---|---|---|
| Freezing Medium: For general cryopreservation of most primary cells. Contains FBS & DMSO. | Size: 50 ml | CAT.#: 040-50 | Price: $54.00 | |
| Cyto-X Cell Counting Reagent: 500 tests | Size: 1 Bottle | CAT.#: 028-01 | Price: $160.00 | |
| Cyto-X Cell Counting Reagent Sample: 100 tests | Size: Sample | CAT.#: 028-S | Price: $41.00 | |
| Cytofect Smooth Muscle Cell Transfection Kit (125 x 24-Wells): 125 x 24-Well | Size: 1 Kit | CAT.#: TF350K | Price: $328.00 | |
| Cytofect Smooth Muscle Cell Transfection Sample Kit (25 x 24-Wells): 25 x 24-Well Rxns | Size: 1 Sample Kit | CAT.#: TF350KS | Price: $62.00 | |
| Subculture Reagent Kit: 100 ml each of HBSS, Trypsin/EDTA & Trypsin Neutralizing Solution | Size: 1 Kit | CAT.#: 090K | Price: $63.00 |
Resources/Documents
Publications
2016
Pugh, R., J. Slee, S. Farwell, Y. Li, T. Barthol, W. Patton and L. Lowe-Krentz. 2016. Transmembrane protein 184A is a receptor required for vascular smooth muscle cell responses to heparin. JBC, 291:5326-5341
Randall, T., S. Islam, I. Mahbub, N. McFarlane and Y. Yu. 2016. A low-power, reconfigurable, pipelined ADC for implantable bioimpedance measurement system with vertically aligned carbon nanofibers (VACNF) electrodes. Analog Integr Circ Sig Process, 89:139-149.
Yu, Y. K. Al Mamun, A. Shanta, S. Islam, and N. McFarlane. 2016. Vertically Aligned Carbon Nanofibers as a Cell Impedance Sensor. IEEE Transactions on Nanotechnology, 10.1109/TNANO.2016.2558102.
2014
Mathura, R., S. Russell-Puleri, L. Cancel, and J. Tarbell. 2014. Hydraulic Conductivity of Endothelial Cell-Initiated Arterial Cocultures. Annals of Biomedical Engineering, 42:763-775.
Slee, J. 2014. Elucidating the Anti-Inflammatory Roles of Heparin and Shear Stress in Atherosclerosis. PhD Dissertation, Lehigh U.
2012
Kuribayashi-Shigetomi, K., H. Onoe, and S. Takeuchi. 2012. Cell origami: self-folding of three-dimensional cell-laden microstructures driven by cell traction force. PloS one. 7:e51085-e51085.
Smela, E. and P. Abshire. 2012. Cell-based sensing: biological transduction of chemical stimuli to electrical signals (nose-on-a-chip). Patent US 8152992 B2.
2011
Dronadula, N., L. Du, R. Flynn, J. Buckler, J. Kho, Z. Jiang, S. Tanaka, and D. Dichek. 2011. Construction of a novel expression cassette for increasing transgene expression in vivo in endothelial cells of large blood vessels. Gene Therapy, 18:501-508.
Patel, H., D. Silversmith, P. Abshire, and T. Datta. 2011. Nose on a Chip, MERIT BIEN 2011 Final Report.
2010
Dronadula, N., L. Du, R. Flynn, J. Buckler, J. Kho, Z. Jiang, S. Tanaka, and D.A. Dichek. 2010. Construction of a novel expression cassette for increasing transgene expression in vivo in endothelial cells of large blood vessels. Gene therapy. 18:501-508.
Fan, L., J. Sakai, S. Bessho, S. Wada, and T. Karino. 2010. Effect of a disturbed flow on adhesion of monocytes to a model of an arterial wall. Biorheology. 47:15-29.
Ohashi, T., N. Kameda, S. Nakamura, and M. Sato. 2010. Biomechanical Contribution of Cytoskeletal Structures to Traction Forces in Cultured Smooth Muscle Cells. J Biomechan Sci and Eng, 5:262:271.
2009
Nelson, N., D. Sander, M. Dandin, S.B. Prakash, A. Sarje, and P. Abshire. 2009. Handheld fluorometers for lab-on-a-chip applications. Biomedical Circuits and Systems, IEEE Transactions on. 3:97-107.
Maier, K.G., B. Sadowitz, S. Cullen, X. Han, and V. Gahtan. 2009. Thrombospondin-1–induced vascular smooth muscle cell migration is dependent on the hyaluronic acid receptor CD44. Am J Surg, 198:664-669.
2008
Du, W. 2008. Mechanics of Aortic Smooth Muscle Cells in Three-dimensional Tissue Constructs. Washington University in St. Luis, PhD dissertation.
Fan, L.J., and T. Karino. 2008. Effect of serum concentration on adhesion of monocytic THP-1 cells onto cultured EC monolayer and EC-SMC co-culture. Journal of Zhejiang University. Science. B. 9:623-629.
Mathews, D.T., Y.A. Birney, P.A. Cahill, and G.B. McGuinness. 2008. Vascular cell viability on polyvinyl alcohol hydrogels modified with water-soluble and -insoluble chitosan. Journal of biomedical materials research. Part B. 84:531-540.
Sakai, J., T. Karino, and K. Niwa. 2008. Flow-dependent accumulation of LDL in co-cultures of endothelial and smooth muscle cells in the presence of filtration flow through the cell layer. Clinical hemorheology and microcirculation. 38:245-256.
Wang, X.-J., K. Maier, S. Fuse, A.I. Willis, E. Olson, S. Nesselroth, B.E. Sumpio, and V. Gahtan. 2008. Thrombospondin-1-induced migration is functionally dependent upon focal adhesion kinase. Vascular and endovascular surgery. 42:256-262.
Yoneyama, T., K. Miyata, T. Chikai, A. Mikami, T. Suzuki, K. Hasegawa, T. Ikeda, T. Watanabe, T. Ohyama, and K. Niwa. 2008. Clostridium botulinum serotype D neurotoxin and toxin complex bind to bovine aortic endothelial cells via sialic acid. FEMS Immunology & Medical Microbiology. 54:290-298.
2007
Prakash, S.B., and P. Abshire. 2007. On-chip capacitance sensing for cell monitoring applications. Sensors J., IEEE. 7:440-447.
2006
Mathews, D.T. 2006. Characterisation of polyvinyl alcohol hydrogels modified with chitosan for cardiovascular applications. Dublin City University, PhD dissertation.
2004
Liu, Y., E. Smela, N.M. Nelson, and P. Abshire. 2004. Cell-lab on a chip: a CMOS-based microsystem for culturing and monitoring cells. In Engineering in Medicine and Biology Society, 2004. IEMBS'04. 26th Annual International Conference of the IEEE. Vol. 1. IEEE. 2534-2537.
Niwa, K., T. Kado, J. Sakai, and T. Karino. 2004. The effects of a shear flow on the uptake of LDL and acetylated LDL by an EC monoculture and an EC–SMC coculture. Ann Biomed Eng. 32:537-543.
Reeves, N., Y. Liu, N.M. Nelson, S. Malhotra, M. Loganathan, J.-M. Lauestein, C. Chaiyupatumpa, E. Smela, and P. Abshire. 2004. Integrated MEMS structures and CMOS circuits for bioelectronic interface with single cells. In Circuits and Systems, 2004. ISCAS'04. Proceedings of the 2004 International Symposium on. Vol. 3. IEEE. III-673-676 Vol. 673.
2002
Gudi, T., J.C. Chen, D.E. Casteel, T.M. Seasholtz, G.R. Boss, and R.B. Pilz. 2002. cGMP-dependent protein kinase inhibits serum-response element-dependent transcription by inhibiting rho activation and functions. J.Biol.Chem. 277:37382-37393.
2001
Sakr, S.W., R.J. Eddy, H. Barth, F. Wang, S. Greenberg, F.R. Maxfield, and I. Tabas. 2001. The uptake and degradation of matrix-bound lipoproteins by macrophages require an intact actin cytoskeleton, Rho family GTPases, and myosin ATPase activity. Journal of Biological Chemistry. 276:37649-37658.
1999
Stathakis, P., A.J. Lay, M. Fitzgerald, C. Schlieker, L.J. Matthias, and P.J. Hogg. 1999. Angiostatin formation involves disulfide bond reduction and proteolysis in kringle 5 of plasmin. Journal of Biological Chemistry. 274:8910-8916.4:8910-8916.