MSDS 211-500
MSDS 211K-500
MSDS 210-500
MSDS 211-GS
Description

Media were optimized for the robust growth and the maintenance of phenotypic and functional characteristics of Endothelial Cells in culture. Media were designed with attention to detail that ensures the consistency of cell health, viability, performance, physiology, morphology and function.
Use with:
- Human Aortic Endothelial Cells: HAOEC
- Human Femoral Artery Endothelial Cells: HFAEC
- Human Pulmonary Artery Endothelial Cells: HPAEC
- Human Umbilical Vein Endothelial Cells: HUVEC
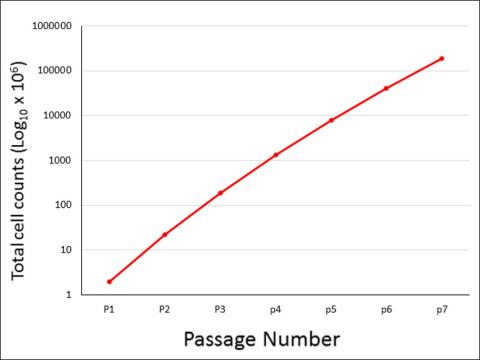
EGM v2 (Cat# 213-500) supports continuous, robust growth
HUVEC were seeded to T25 flasks, passaged repeatedly, and counted for cell number.
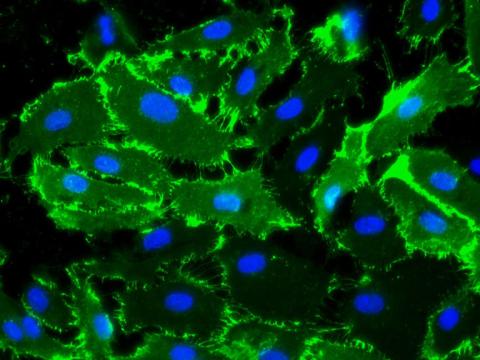
Sustained expression of endothelial cell markers
Growth of primary Human Aortic Endothelialial Cells (HAOEC) in EGM (Cat# 211-500) maintains CD31 expression.
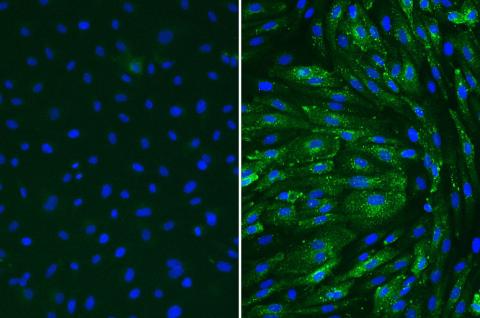
Following treatment of TNF-alpha, Human Coronary Artery Endothelial cells (HCAEC) upregulate VCAM-1 (right). Untreated (left)
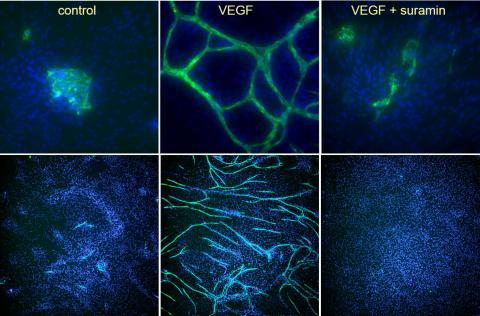
For 10 days, cells were stimulated with VEGF or VEGF in the presence of the angiogenesis inhibitor suramin.
Transfect primary endothelial cells with Cytofect Transfection Kits
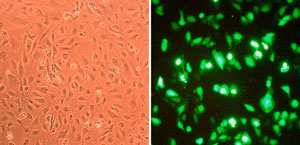
Convenient, non-toxic transfection of HAOEC with GFP plasmid.
According to Sara Lynn Farwell, Ph.D. Candidate, Cell & Molecular Bio. at Lehigh University: “CAI’s Endothelial Cell Growth Medium is reliable for our experiments, as well as general culturing. There must be magic in their media, because when we had problems with other formulations, the CAI product fixed the issues!”
Note that endothelial Cell Defined Medium is designed for maintaining the monolayer of endothelial cells at 100% confluent culture. It does not contain the growth factors VEGF, FGF or PDGF, nor does it contain serum, but is instead supplemented with defined components to allow metabolic and growth factor studies without interference of extraneous factors.
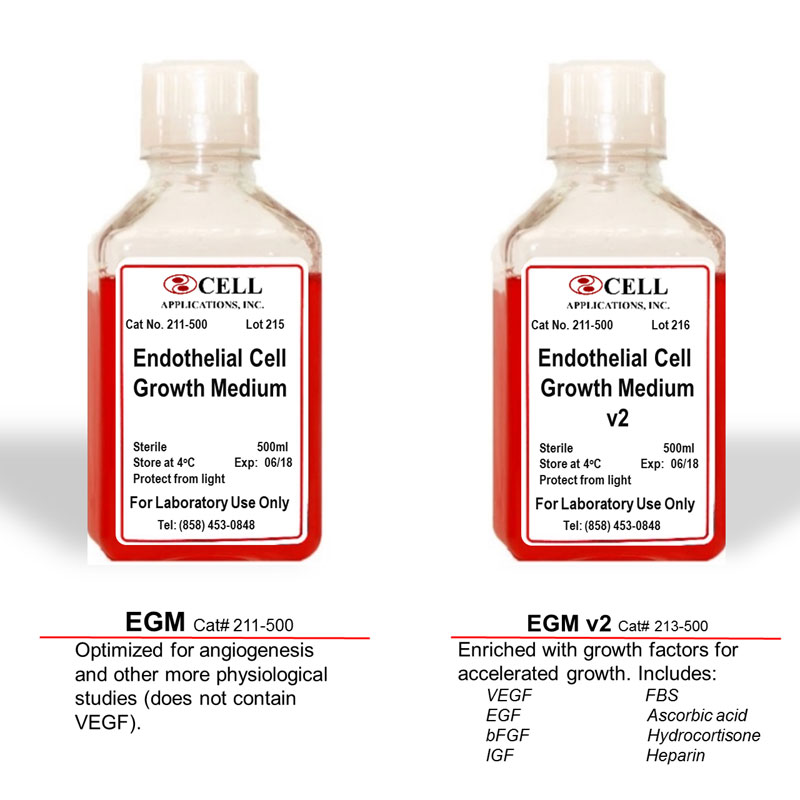
Choose the Human Endothelial Cell Medium best suited for your specific applications. Optimized for in vitro culture of a variety of Endothelial Cells from aorta, artery and vein. EGM (Cat# 211-500) is optimized for angiogenesis and other more physiological studies (does not contain VEGF). EGM v2 (Cat# 213-500) is enriched with growth factors for accelerated growth, and includes VEGF, FBS, EGF, bFGF, IGF, Ascorbic acid, Hydrocortisone, Heparin.
Details
CAI media are tested for sterility in order to confirm no bacteria, yeast or fungi contaminate the solutions. The products undergo further quality control for correct pH, osmolality and lack of endotoxins. A panel of different bioassays affirm the media sustain a proper environment for expected cell-type-specific culture, growth, plating, karyotype, physiology, morphology, viability, population doublings, surface markers, cryopreservation, differentiation and/or induction.
Laboratory research use only (RUO). Not for human, clinical, diagnostic or veterinary use.
Products
| Product | Size | CAT.# | Price | Quantity |
|---|---|---|---|---|
| Human EC Growth Medium: All-in-one ready-to-use. Optimized and best-suited for angiogenesis and other physiological studies. For accelerated growth, use Cat# 213-500. | Size: 500 ml | CAT.#: 211-500 | Price: $121.00 | |
| Human EC Growth Medium Kit: Basal medium & growth supplement sold together packaged separately. Optimized and best-suited for angiogenesis and other physiological studies. For accelerated growth, use Cat# 213-500. | Size: Yields 500 ml | CAT.#: 211K-500 | Price: $143.00 | |
| Human EC Basal Medium: Basal medium (contains no growth supplement). Add GS before use. | Size: 500 ml | CAT.#: 210-500 | Price: $91.00 | |
| Human EC Growth Supplement: Added to Basal Medium to create Growth Medium. The complete Growth Medium (equivalent to Cat# 211-500) is optimized and best-suited for angiogenesis and other physiological studies. For accelerated growth, use Cat# 213-500. | Size: 15 ml | CAT.#: 211-GS | Price: $74.00 | |
| Human EC Growth Medium V2: All-in-one ready-to-use. Enriched with more growth factors for accelerated cell growth. For angiogenesis studies, use Cat# 211-500. | Size: 500 ml | CAT.#: 213-500 | Price: $141.00 | |
| Endothelial Cells Growth Medium V2 Kit, 500ml: Basal medium & growth supplement sold together packaged separately. Enriched with more growth factors for accelerated cell growth. For angiogenesis studies, use Cat# 211K-500. | Size: 500 ml | CAT.#: 213K-500 | Price: $144.00 | |
| Human EC Growth Medium Kit V2, components separate: Basal medium & growth supplement sold together packaged separately. Supplements in individual vials. Enriched with more growth factors for accelerated cell growth. For angiogenesis studies, use Cat# 211K-500. | Size: 500 ml | CAT.#: 213KS-500 | Price: $155.00 |
Related Products
| Product | Size | CAT.# | Price | Quantity |
|---|---|---|---|---|
| Human EC Serum-Free Defined Medium: Defined medium without serum designed to maintain endothelial cell monlayers in confluent cultures. Not for cell growth. | Size: 500 ml | CAT.#: 113-500 | Price: $130.00 | |
| Human EC Serum-Free Defined Medium wo Phenol Red: Defined medium, without serum or phenol red, designed to maintain endothelial cell monlayers in 100% confluent culture. Not intended for cell growth. | Size: 500 ml | CAT.#: 113PR-500 | Price: $136.00 | |
| Human EC Growth Medium wo Antibiotics: Growth medium without antibiotics. Optimized and best-suited for angiogenesis and other physiological studies. Suitable for Cytofect Transfection Kit. For accelerated growth, use Cat# 213-500. | Size: 500 ml | CAT.#: 211A-500 | Price: $130.00 | |
| Human EC Growth Medium wo FBS: 211-500 growth medium, but without FBS. FBS must be added in order to support cell culture. | Size: 500 ml | CAT.#: 211F-500 | Price: $130.00 | |
| Human EC Growth Medium wo Hydrocortisone: Growth medium without hydrocortisone. The complete Growth Medium is optimized and best-suited for angiogenesis and other physiological studies. For accelerated growth, use Cat# 213-500. | Size: 500 ml | CAT.#: 211H-500 | Price: $130.00 | |
| Human EC Growth Medium wo Phenol Red: Growth medium without phenol red. Optimized and best-suited for angiogenesis and other physiological studies. For accelerated growth, use Cat# 213-500. | Size: 500 ml | CAT.#: 211PR-500 | Price: $130.00 | |
| Human EC Basal Medium wo Phenol Red: Basal medium without growth supplement and phenol red | Size: 500 ml | CAT.#: 210PR-500 | Price: $98.00 | |
| Human EC Growth Supplement wo Antibiotics: Growth supplement without antibiotics | Size: 15 ml | CAT.#: 211A-GS | Price: $88.00 | |
| Human EC Growth Supplement wo FBS: Growth supplement without FBS. FBS must be added in order to support cell culture. | Size: 5 ml | CAT.#: 211F-GS | Price: $91.00 | |
| Human EC Growth Supplement wo Hydrocortisone: Growth supplement without hydrocortisone | Size: 15 ml | CAT.#: 211H-GS | Price: $88.00 | |
| Human EC Growth Medium V2 without FBS: Endothlelial Cell Growth Medium v2, without FBS. | Size: 500 ml | CAT.#: 213F-500 | Price: $153.00 | |
| Human EC Starvation Medium: Use when cells need to be starved overnight to 24 hrs before experiment | Size: 250 ml | CAT.#: 209-250 | Price: $81.00 | |
| Human EC Starvation Medium wo Phenol Red: Starvation medium without phenol red | Size: 250 ml | CAT.#: 209PR-250 | Price: $91.00 |
Extended Family Products
| Product | Size | CAT.# | Price | Quantity |
|---|---|---|---|---|
| Cyto-X Cell Counting Reagent Sample: 100 tests | Size: Sample | CAT.#: 028-S | Price: $41.00 | |
| Cyto-X Cell Counting Reagent: 500 tests | Size: 1 Bottle | CAT.#: 028-01 | Price: $160.00 | |
| Cytofect Endothelial Cell Transfection Kit (250 x 24-Wells): 250 x 24-Well Rxns | Size: 1 Kit | CAT.#: TF101K | Price: $496.00 | |
| Cytofect Endothelial Cell Transfection Sample Kit (25 x 24-Wells): 25 x 24-Well Rxns | Size: 1 Sample Kit | CAT.#: TF101KS | Price: $62.00 | |
| Freezing Medium: For general cryopreservation of most primary cells. Contains FBS & DMSO. | Size: 50 ml | CAT.#: 040-50 | Price: $54.00 | |
| Subculture Reagent Kit: 100 ml each of HBSS, Trypsin/EDTA & Trypsin Neutralizing Solution | Size: 1 Kit | CAT.#: 090K | Price: $63.00 | |
| HUVEC Conditioned Medium: HUVEC Conditioned Medium | Size: 5 ml | CAT.#: 211CM-05 | Price: $55.00 |
Resources/Documents
Publications
2017
Tan, W., Wang, J., Zhou, F., Gao, L., Rong, Y., Liu, H., Sukanthanag, A., Wang, G., Mihm, M.C., Chen, D.B. and Nelson, J.S., 2017. Coexistence of EphB1 and EphrinB2 in Port Wine Stain Endothelial Progenitor Cells Contributes to Clinicopathological Vasculature Dilatation. British Journal of Dermatology. DOI: 10.1111/bjd.15716.
Shatanawi, A. and Momani, M.S., 2017. Plasma arginase activity is elevated in type 2 diabetic patients. Biomedical Research, 28(9).
Y Xue, CS Hilaire, L Hortells, JA Phillippi, V Sant, S Sant. 2017. Shape-Specific Nanoceria Mitigate Oxidative Stress-Induced Calcification in Primary Human Valvular Interstitial Cell Culture. Cellular and Molecular Bioengineering, 1-18.
Fercana, G., S. Yerneni, M. Billaud, J. Hill, P. VanRyzin, T. Richards, B. Sicari, S. Johnson, S. Badylak, P,. Campbell, T. GLEASON and J. Phillippi. 2017. Perivascular extracellular matrix hydrogels mimic native matrix microarchitecture and promote angiogenesis via basic fibroblast growth factor. Biomaterials, 123:142-154.
Gardner, A., P. Montgomery, Y. Zhao, F. Silva-Palacios, Z. Ungvari, A. Csiszar and W. Sonntag. 2017. Association between daily walking and antioxidant capacity in patients with symptomatic peripheral artery disease. J Vasc Surg, dx.doi.org/10.1016/j.jvs.2016.12.108.
Xue, Y, T. Yatsenko, A. Patel, D. Stolz, J. Philippi, V. Sant and S. Sant. 2017. PEGylated poly(ester amide) elastomer scaffolds for soft tissue engineering. Polymers for Adv Tech, DOI: 10.1002/pat.4002
2016
Benza, R., G. Lentz, C. Wu, K. Shields, A. Raina, S. Murali, and M. Passineau. 2016. In Situ expression of bcl-2 in pulmonary artery endothelial cells associated with pulmonary arterial hypertension relative to heart failure with preserved ejection fraction. Pulm Circ, 6:551-556.
Cao, Y., M. Roursgaard, N. Jacobsen, P. Møller and S. Loft. 2016. Monocyte adhesion induced by multi-walled carbon nanotubes and palmitic acid in endothelial cells and alveolar–endothelial co-cultures. 235-244.
Chiang, E. H. Ma, J. Wang, C. Liu, T. Chen andn S. Hung. 2016. Multi-lineage differentiation and angiogenesis potentials of pigmented villonodular synovitis derived mesenchymal stem cells - pathological implication. J Orth Res, 34:395-403.
Ganta, V., M. Choi, A. Kutateladze and B. Annex. 2016. VEGF165b Modulates Endothelial VEGFR1-STAT3 Signaling Pathway and Angiogenesis in Human and Experimental Peripheral Arterial Disease. Circulation Research, DOI: 10.1161/CIRCRESAHA.116.309516.
Jia, P., H. Chen, H. Kang, J. Qi, P. Zhao, M. Jiang, L. Guo, Q. Zhou, N. Qian, H. Zhou, Y. Xu, Y. Fan and L. Deng. 2016. Deferoxamine released from poly(lactic-co-glycolic acid) promotes healing of osteoporotic bone defect via enhanced angiogenesis and osteogenesis. J Biomed Mat Res 104:2515-2527.
Lamichhane, S., J. Anderson, T. Remund, H. Sun, M. Larson, P. Kelly and G. Mani. 2016. Responses of endothelial cells, smooth muscle cells, and platelets dependent on the surface topography of polytetrafluoroethylene. J Biomed Mat Res, 104:2291-2304.
Lansford, K., D. Shill, A. Dicks, M. Marshburn, W. Southern. And N. Jenkins. 2016. Effect of acute exercise on circulating angiogenic cell and microparticle populations. Experimental Physiology, 101:155.
Nymo, S., A. Gustavsen, P. Nilsson, C. Lau, T. Espevik and T. Mollnes. 2016. Human Endothelial Cell Activation by Escherichia coli and Staphylococcus aureus Is Mediated by TNF and IL-1β Secondarily to Activation of C5 and CD14 in Whole Blood. J Immunol, 196:2293-2299.
Ramírez-Sánchez, I., A. Rodríguez, A. Moreno-Ulloa, G. Ceballos and F. Villarreal. 2016. (-)-Epicatechin-induced recovery of mitochondria from simulated diabetes: Potential role of endothelial nitric oxide synthase. Diabetes & Vasc Dis Res, 13:201-210.
Shi, L., A. Kim, R. Chang, J. Chang, W. Ying, M. Ko, B. Zhou and G. Ko. 2016. Deletion of miR-150 Exacerbates Retinal Vascular Overgrowth in High-Fat-Diet Induced Diabetic Mice. PLoS ONE, 11(6): e0157543.
Wang, H., S. Chen and W. Lo. 2016. Identification of Cofilin-1 Induces G0/G1 Arrest and Autophagy in Angiotensin-(1-7)-treated Human Aortic Endothelial Cells from iTRAQ Quantitative Proteomics. Scientific Reports, 6, 35372.
Wang, Y. W. Nie, K. Yao, Z. Wang andn H. He. 2016. Interleukin 6 induces expression of NADPH oxidase 2 in human aortic endothelial cells via long noncoding RNA MALAT1. Die Pharmazie, 71:592-597.
Yuana, M., T. Wangb, B. Konga, X. Wanga, C. Huanga and D. Wangb. 2016. GHSR-1a is a novel pro-angiogenic and anti-remodeling target in rats after myocardial infarction. Eur J Pharmacol, 788:218-225.
Zhaocheng, J., L. Jinfeng, Y. Luchang, S. Yequan, L. Feng and W. Kai. 2016. Ginkgolide A inhibits lipopolysaccharide-induced inflammatory response in human coronary artery endothelial cells via downregulation of TLR4-NF-κB signaling through PI3K/Akt pathway. Die Pharmazie, 71:588-591.
2015
Anderson, J., T. Remund, K. Pohlson, S. Lamichhane, C. Evans, R. Evans, M. Clark, K. Egland, P. Kelly and G. Mani. 2015. In vitro and in vivo evaluation of effect of excipients in local delivery of paclitaxel using microporous infusion balloon catheters. J Biomed Mater Res Part B, DOI: 10.1002/jbm.b.33564.
Annex, B., C. Farber, and S. Hazarika. 2015. COMPOSITIONS AND METHODS FOR TREATING PERIPHERAL ARTERIAL DISEASE. Patent Application US 20150218556 A1.
Chang, E., H. Ma, J. Wang, C. Liu, T. Chen, and S Hung. 2015. Multi-lineage differentiation and angiogenesis potentials of pigmented villonodular synovitis derived mesenchymal stem cells - pathological implication. J Orthopaedic Res, DOI: 10.1002/jor.23031.
Chou, C., S. Lai, C. Ho, W. Lin, C. Chen, P. Lee, F. Peng, S. Kuo, S. Wu and H. Lai. 2015. Lysophosphatidic Acid Alters the Expression Profiles of Angiogenic Factors, Cytokines, and Chemokines in Mouse Liver Sinusoidal Endothelial Cells. PLoS ONE 10(3): e0122060.
Danielsen, P., Y. Cao, M. Roursgaard, P. Møller & S. Loft. 2015. Endothelial cell activation, oxidative stress and inflammation induced by a panel of metal-based nanomaterials. Nanotoxicology 9:813-824.
Devapatla, B., A. Sharma and S. Woo. 2015. CXCR2 Inhibition Combined with Sorafenib Improved Antitumor and Antiangiogenic Response in Preclinical Models of Ovarian Cancer. PLoS ONE 10(9): e0139237.
Dokun, A., L. Chen, M. Okutsu, C. Farber, S. Hazarika, W. Jones, D. Craig, D. Marchuk, J. Lye, S. Shan, and B. Annex. 2015. ADAM12: A Genetic Modifier of Pre-clinical Peripheral Arterial Disease. Am J Physiol, DOI: 10.1152/ajpheart.00803.2014.
Flebus, L., F. Lombart, C. Sevrin, J. Defraigne, P. Peters, L. Parhamifar, D. Molin and C. Grandfils. 2015. Low molecular weight poly (2-dimethylamino ethylmethacrylate) polymers with controlled positioned fluorescent labeling: Synthesis, characterization and in vitro interaction with human endothelial cells. Int J Pharmaceutics, 478:278-287.
Gardner, A., D. Parker, P. Montgomery, D. Sosnowska, A. Casanegra, Z. Ungvari, A. Csiszar, S. Zhang, J. Wang and W. Sonntag. 2015. Influence of diabetes on ambulation and inflammation in men and women with symptomatic peripheral artery disease. J Clin & Translational Endocrinol, 2:137-143.
He, J. and Y, Li. 2015. Ginsenoside Rg1 Downregulates the Shear Stress Induced MCP-1 Expression by Inhibiting MAPK Signaling Pathway. Am J Chin Med, 43:305.
Huang, C., G. Li, H. Dong, S. Sun, H. Chen, D. Luo, L. Sun, X. Li, Z. Chen, H. Yang, S. Wei, and Y. Zhou. 2015. Arg972 insulin receptor substrate-1 inhibits endothelial nitric oxide synthase expression in human endothelial cells by upregulating microRNA-155. International Journal of Molecular Medicine, 36:239-248.
Izaguirre-Carbonell, J., H. Kawakubo, H. Murata, A. Tanabe, T. Takeuchi, T. Kusayanagi, S. Tsukuda, T. Hirakawa, K. Iwabata, Y. Kanai, K. Ohta, M. Miura, K. Sakaguchi, S. Matsunaga, H. Sahara, S. Kamisuki and F. Sugawara. 2015. Novel anticancer agent, SQAP, binds to focal adhesion kinase and modulates its activity. Sci Rep, 5:15136.
Klingberg, H., L. Oddershede, S. Loft, and P. Møller. 2015. Influence of flow, shear stress and adhesion molecule targeting on gold nanoparticle uptake in human endothelial cells. Nanoscale, DOI: 10.1039/C5NR01467K.
Lansford, K., D. Shill, A. Dicks, M. Marshburn, W. Southern. And N. Jenkins. 2015.Effect of acute exercise on circulating angiogenic cell and microparticle populations. Experimental Physiology, doi: 10.1113/EP085505.
Liu, G., B. Liang, W. Lau, Y. Wang, J. Zhao, R. Li, X. Wang, Y. Yuan, B. Lopez, T. Christopher, C. Xiao, X. Ma and Y. Wang. 2015. High glucose/High Lipids impair vascular adiponectin function via inhibition of caveolin-1/AdipoR1 signalsome formation. Free Radical Biol & Med, 89:473-485.
Madasamy, S. 2015. Multi-subunit biological complexes for treatment of plaque-associated diseases. Patent US8932558B2.
Nelson, J. 2015. Dynamic Blood Flow Modulates Endothelial Mitochondrial Redox States and Vascular Repair. PhD Dissertation, UCLA.
Nishitani, W., A. Alencar and Y. Wang. 2015. Rapid and Localized Mechanical Stimulation and Adhesion Assay: TRPM7 Involvement in Calcium Signaling and Cell Adhesion. PLoS ONE, 10(5): e0126440.
Passineau, M., S. Murali, R. Benza, and J. Pollett. 2015. ISOLATION OF PULMONARY ARTERIAL ENDOTHELIAL CELLS FROM PATIENTS WITH PULMONARY VASCULAR DISEASE AND USES THEREOF. Patent Application US 20150219631 A1.
Tsai, I., C. Chou, Y. Yang, W. Lin, Y. Lin, L. Chow, H. Lee, P. Kao, W. Liau, T. Jou and Y. Tsau. 2015. Inhibition of Rho-associated kinase relieves C5a-induced proteinuria in murine nephrotic syndrome. Cell & Molec Life Sci, 72:3157-3171.
Zelko, I. and R. Folz. 2015. Regulation of Oxidative Stress in Pulmonary Artery Endothelium. Modulation of Extracellular Superoxide Dismutase and NOX4 Expression Using Histone Deacetylase Class I Inhibitors. Am J Resp Cell & Molec Biol, 53:513-524.
2014
Cao, Y., N. Jacobsen, P. Danielsen, A. Lenz, T. Stoeger, S. Loft, H. Wallin, M. Roursgaard, L. Mikkelsen and P. Møller. 2014. Vascular Effects of Multiwalled Carbon Nanotubes in Dyslipidemic ApoE−/− Mice and Cultured Endothelial Cells. Toxicol Sci, 138:104-116.
Cao, Y., M. Roursgaard, P. Danielsen, P. Møller and S. Loft. 2014. Carbon Black Nanoparticles Promote Endothelial Activation and Lipid Accumulation in Macrophages Independently of Intracellular ROS Production. PLoS ONE, dx.doi.org/10.1371/journal.pone.0106711.
Hwang, J., H. Lim, C. Yoon, K. Lam, S. Yoon, C. Lee, C. Chiu, B. Kang, H. Kim and K. Shung. 2014. Non-contact High-Frequency Ultrasound Microbeam Stimulation for Studying Mechanotransduction in Human Umbilical Vein Endothelial Cells. Ultrasound in Med & Biol, 40:2172-2182.
Kaakinen, M., S. Huttenen, L. Paavolainen, V. Marjomaki, J. Heikkila, and L. Eklund. 2014. Automatic detection and analysis of cell motility in phase-contrast time-lapse images using a combination of maximally stable extremal regions and Kalman filter approaches. J Microscopy, 253:65-78.
Li, C., M. Mpollo, C. Gonsalves, S. Tahara, P. Malik and V. Kalra. 2014. Peroxisome Proliferator-activated Receptor-α-mediated Transcription of miR-199a2 Attenuates Endothelin-1 Expression via Hypoxia-inducible Factor-1α. J Biol Chem, 289:36031-36047.
Sasahira, T., T. Kirita, K. Yamamoto, N. Ueda, M. Kurihara, S. Matsushima, U. Bhawal, A. Bosserhoff and H. Kuniyasu. 2014. Transport and Golgi organisation protein 1 is a novel tumour progressive factor in oral squamous cell carcinoma. Eur J Cancer, 50-2142-2151.
Sasahira, T. N. Ueda, K. Yamamoto, M. Kurihara, S. Matsushima, U. Bhawal, T. Kirita and H. Kuniyasu. 2014. Prox1 and FOXC2 Act as Regulators of Lymphangiogenesis and Angiogenesis in Oral Squamous Cell Carcinoma. PLoS ONE, dx.doi.org/10.1371/journal.pone.0092534.
Singh, V. 2014. Treatment of endometriosis, angiogenesis and/or endometrial lesion growth. Patent Application US 20160038561 A1.
Teramatsu, Y., H. Maeda, H. Sugii, A. Tomokiyo, S. Hamano, N. Wada, A. Yuda, N. Yamamoto, K. Koori and A. Akamine. 2014. Expression and effects of epidermal growth factor on human periodontal ligament cells. Cell & Tiss Res, 357:633-643.
Thakali, K., J. Saben, J. Faske, F. Lindsey, H. Gomez-Acevedo, C. Lowery, T. Badger A. Andres and K. Shankar. 2014. Maternal pregravid obesity changes gene expression profiles toward greater inflammation and reduced insulin sensitivity in umbilical cord. Pediatric Res, 76:202-210.
Wang, X., A. Zachman, Y. Chun, F. Shen, Y. Hwang and H. Sung. 2014. Polymeric stent materials dysregulate macrophage and endothelial cell functions: Implications for coronary artery stent.
Intl J Cardiol, 174:688-695.
2012
Abe, H. and S. Tajima. 2012. UVB irradiation down-regulates type XVI collagen expression in mouse and human skin. J Cosmetic Dermatol, 11:169-178.
Baumgartner-Parzer, S.M., F.R. Waldenberger, A. Freudenthaler, A. Ginouvès-Guerdoux, D. McGahie, and H. Gatto. 2012. The Natural Antioxidants, Pomegranate Extract and Soy Isoflavones, Favourably Modulate Canine Endothelial Cell Function. ISRN veterinary science. 2012: doi:590310.595402/592012/590328.
Bernson, E. 2012. Development of a Microfluidic Platform for Cell migration Studies along Gradients. In Department of Applied Physics. MS Thesis, Chalmers University of Technology.
Cossu, A. 2012. Study of intracellular signaling pathways triggered by natural antioxidants in human endothelial cells. Doctoral Thesis, Università degli studi di Sassari.
Ding, S., D.M. Pinkas, and A.E. Barron. 2012. Synthesis and assembly of functional high molecular weight adiponectin multimers in an engineered strain of Escherichia coli. Biomacromolecules. 13:1035-1042.
Donneys, A., D.M. Weiss, S.S. Deshpande, S. Ahsan, C.N. Tchanque-Fossuo, D. Sarhaddi, B. Levi, S.A. Goldstein, and S.R. Buchman. 2012. Localized deferoxamine injection augments vascularity and improves bony union in pathologic fracture healing after radiotherapy. Bone. 52:318–325.
Eid, N. 2012. FSTL-1 SECRETED BY MESENCHYMAL STEM CELLS INCREASES CELL VIABILITY OF HUMAN AORTIC ENDOTHELIAL CELLS UNDER HYPOXIC STRESS. BA Thesis, Wilkes Honors College of Florida Atlantic University.
Forchhammer, L., S. Loft, M. Roursgaard, Y. Cao, I.S. Riddervold, T. Sigsgaard, and P. Møller. 2012. Expression of adhesion molecules, monocyte interactions and oxidative stress in human endothelial cells exposed to wood smoke and diesel exhaust particulate matter. Toxicology letters. 209:121-128.
Giedt, R. 2012. Mitochondrial Network Dynamics in Vascular Endothelial Cells Exposed to Mechanochemical Stimuli: Experimental and Mathematical Analysis. PhD Dissertation, Ohio State U.
Giedt, R., D. Pfeiffer, A. Matzavinos, C. Kao and B. Alevriadou. 2012. Mitochondrial Dynamics and Motility Inside Living Vascular Endothelial Cells: Role of Bioenergetics. Annals of Biomed Eng, 40:1903-1916.
Giedt, R., C. Yang, J. Zweier, A. Matzavinos and B. Alevriadou. 2012. Mitochondrial fission in endothelial cells after simulated ischemia/reperfusion: role of nitric oxide and reactive oxygen species. Free Rad Biol & Med, 52:348-356.
Hankins, J. 2012. Re-branding ceramide-1-phosphate: Not just a ceramide metabolite. PhD Dissertation, Penn State U.
He, J., Y. Li, X. Yang, X. He, H. Zhang, J. He and L. Zhang. 2012. The Feedback Regulation of PI3K-miR-19a, and MAPK-miR-23b/27b in Endothelial Cells under Shear Stress. Molecules 18:1-13.
Leijnse, N. J. Jeon, S. Loft, R. Metzler, and L. Oddershede. 2012. Diffusion inside living human cells. Eu Phys J Special Topics, 1:75-84.
Lin, L.-Y., H.-Y. Lin, H.-W. Chen, T.-L. Su, L.-C. Huang, and K.-J. Chuang. 2012. Effects of temple particles on inflammation and endothelial cell response. Science of The Total Environment. 414:68-72.
Monginoux, P. H. Gatto, C. Karst, and F. Waldenberger. 2012. Products for oral administration comprising extracts of punica granatum (pomegranate), intended for a pet, and applications of same. Patent Application US 20140335180 A1.
Murai, A., S. Asa, A. Kodama, A. Hirata, T. Yanai, and H. Sakai. 2012. Constitutive phosphorylation of the mTORC2/Akt/4E-BP1 pathway in newly derived canine hemangiosarcoma cell lines. BMC Veterinary Research. 8:128.
Naughton, G., J. Mansbridge, R. Pinney, and J. Zeltinger. 2012. Methods for using a three-dimensional stromal tissue to promote angiogenesis. Patent US 8128924 B2.
Nakamura, D., A. Edwards, S. Virani, R. Thomas and C. Tayade. 2012. Thrombospondin-1 Mimetic Peptide ABT-898 Affects Neovascularization and Survival of Human Endometriotic Lesions in a Mouse Model. Am J Pathol, 181:570-582.
Osterbur, K. 2012. The mechanism of C-type natriuretic peptide production in dogs and its use as a prognostic indicator in critically ill dogs. University of Missouri, MSc dissertation.
Othumpangat, S., C. Walton, and G. Piedimonte. 2012b. MicroRNA-221 Modulates RSV Replication in Human Bronchial Epithelium by Targeting NGF Expression. PloS one. 7:e30030.
Paolillo, R., M. Lovene, C. Carratelli, and A. Rizzo. 2012. Induction of VEGF and MMP-9 Expression by Toll-like Receptor 2/4 in Human Endothelial Cells Infected with Chlamydia Pneumoniae. Intl J Immunopathol & Pharmacol, 25:377-386.
Soenen, S., M. Cuyper, S. De Smedt, and K. Braeckmans. Investigating the toxic effects of iron oxide nanoparticles. Chapter 10. In Duzgunes, N. 2012. Nanomedicine: Infectious Diseases, Immunotherapy, Diagnostics, Antifibrotics, Toxicology and Gene Medicine. Methods in Enzymology, Vol 509.
Takino, J., S. Yamagishi, and M. Takeuchi. 2012. Glycer-AGEs-RAGE signaling enhances the angiogenic potential of hepatocellular carcinoma by upregulating VEGF expression. World journal of gastroenterology: WJG. 18:1781.
Tsuji, T., H. Yoshitomi and J. Usukura. 2012. Endocytic mechanism of transferrin-conjugated nanoparticles and the effects of their size and ligand number on the efficiency of drug delivery. J Electron Microsc, doi: 10.1093/jmicro/dfs080.
Wang, H.-J., H.-C. Huang, Y.-C. Chuang, P.-J. Liao, D.-M. Yang, W. Yang, and H. Huang. 2012. Modulation of tissue factor and thrombomodulin expression in human aortic endothelial cells incubated with high glucose. Acta Diabetol. 49:125-130.
Wang, K. 2012. The role of microRNAs in flow regulation of endothelial functions. PhD Dissertation, UCSD.
Wang, X., Z. Zhang, and C. Yao. 2012. Bortezomib Inhibits the Angiogenesis Mediated by Mesenchymal Stem Cells. Cellular and Molec Biol, 30:657-662.
Yeh, Y., S. Hur, J. Chang, K. Wang, J. Chiu, Y. Li and S. Chien. 2012. Matrix Stiffness Regulates Endothelial Cell Proliferation through Septin 9. PLoS ONE, dx.doi.org/10.1371/journal.pone.0046889.
2011
Konakahara, S., M. Saitou, S. Hori, T. Nakane, K. Murai, R. Itoh, A. Shinsaka, J. Kohroki, T. Kawakami, M. Kajikawa, and Y. Masuho. 2011. A neuronal transmembrane protein LRFN4 induces monocyte/macrophage migration via actin cytoskeleton reorganization. FEBS letters. 585:2377-2384.
Mikkelsen, L., M. Sheykhzade, K.A. Jensen, A.T. Saber, N.R. Jacobsen, U. Vogel, H. Wallin, S. Loft, and P. Moller. 2011. Modest effect on plaque progression and vasodilatory function in atherosclerosis-prone mice exposed to nanosized TiO2. Part Fibre Toxicol. 8:32.
Mukai, R., H. Ashida, J. Terao, and N. Saito. 2011. Determination of Subcellular Localization of Flavonol in Cultured Cells by Laser Scanning. In Laser Scanning, Theory and Applications. C. Wang (Ed.), ISBN: 978-953-307-205-0.
Sharma, V., and H.H. Freeze. 2011a. Mannose Efflux from the Cells: A POTENTIAL SOURCE OF MANNOSE IN BLOOD. Journal of Biological Chemistry. 286:10193-10200.
Sharma, V., M. Ichikawa, P. He, Y. Bravo, R. Dahl, B. Ng, N. Cosford, and H. Freeze. 2011. Phosphomannose Isomerase Inhibitors Improve N-Glycosylation in Selected Phosphomannomutase-deficient Fibroblasts. JCB, 286:39431-39438.
Shatanawi, A., M.J. Romero, J.A. Iddings, S. Chandra, N.S. Umapathy, A.D. Verin, R.B. Caldwell, and R.W. Caldwell. 2011. Angiotensin II-induced vascular endothelial dysfunction through RhoA/Rho kinase/p38 mitogen-activated protein kinase/arginase pathway. American Journal of Physiology - Cell Physiology. 300:C1181-C1192.
Wang, H.-J., T.-L. Lu, H. Huang, and H.-C. Huang. 2011. Paclitaxel induces thrombomodulin downregulation in human aortic endothelial cells. Texas Heart Institute Journal. 38:20.
Winkles, J., and M. Yepes. 2011. TWEAK as a therapeutic target for treating central nervous system diseases associated with cerebral edema and cell death. Patent US 7939490 B2.
Xu, H., M. Zaidi, J. Struve, D.W. Jones, J.G. Krolikowski, S. Nandedkar, N.L. Lohr, A. Gadicherla, P.S. Pagel, M.E. Csuka, K.A. Pritchard, and D. Weihrauch. 2011. Abnormal fibrillin-1 expression and chronic oxidative stress mediate endothelial mesenchymal transition in a murine model of systemic sclerosis. American Journal of Physiology - Cell Physiology. 300:C550-C556.
Yang, C.J., C.Y. Lin, T.-c. Hsieh, S.C. Olson, and J.M. Wu. 2011. Control of eotaxin-1 expression and release by resveratrol and its metabolites in culture human pulmonary artery endothelial cells. American journal of cardiovascular disease. 1:16.
Yew, T.-L., Y.-T. Hung, H.-Y. Li, H.-W. Chen, L.-L. Chen, K.-S. Tsai, S.-H. Chiou, K.-C. Chao, T.-F. Huang, H.-L. Chen, and S.-C. Hung. 2011. Enhancement of Wound Healing by Human Multipotent Stromal Cell Conditioned Medium: The Paracrine Factors and p38 MAPK Activation. Cell Transplantation. 20:693-706.
Yoshikawa, A., Y. Aizaki, K.-i. Kusano, F. Kishi, T. Susumu, S. Iida, S. Ishiura, S. Nishimura, M. Shichiri, and T. Senbonmatsu. 2011. The (pro)renin receptor is cleaved by ADAM19 in the Golgi leading to its secretion into extracellular space. Hypertension research. 34:599-605.
2010
Hung, C.-H., D. Wu, F.-Y. Lin, R.-Y. Yuan, and C.-J. Hu. 2010. Toll-like Receptor 4 and Vascular Cell Adhesion Molecule 1 in Monocyte-Endothelium Adhesion Induced by Lipopolysaccharide. J. Experimental & Clinical Medicine. 2:297-301.
Blasberg, J.D., C.M. Goparaju, H.I. Pass, and J.S. Donington. 2010. Lung cancer osteopontin isoforms exhibit angiogenic functional heterogeneity. The Journal of Thoracic and Cardiovascular Surgery. 139:1587-1593.
Christensen, J. and Y. Zou. 2010. Method of treating abnormal cell growth. Patent US 7825137 B2.
Gelissen, I.C., S. Cartland, A.J. Brown, C. Sandoval, M. Kim, D.L. Dinnes, Y. Lee, V. Hsieh, K. Gaus, L. Kritharides, and W. Jessup. 2010. Expression and stability of two isoforms of ABCG1 in human vascular cells. Atherosclerosis. 208:75-82.
Hou, X., J. Song, X.-N. Li, L. Zhang, X. Wang, L. Chen, and Y.H. Shen. 2010. Metformin reduces intracellular reactive oxygen species levels by upregulating expression of the antioxidant thioredoxin via the AMPK-FOXO3 pathway. Biochemical and biophysical research communications. 396:199-205.
Li, R., Z. Ning, J. Cui, F. Yu, C. Sioutas, and T. Hsiai. 2010. Diesel exhaust particles modulate vascular endothelial cell permeability: Implication of ZO-1 expression. Toxicology letters. 197:163-168.
Miyake, Y., M. Mochizuki, C. Ito, M. Itoigawa, and T. Osawa. 2010. Peroxynitrite Scavengers Produced by Filamentous Fungus Used in the Katsuobushi Manufacturing Process. Food science and technology research. 16:493-498.
Mochizuki, M., Y. Tsuchie, N. Yamada, Y. Miyake, and T. Osawa. 2010. Effect of sesame lignans on TNF-α-induced expression of adhesion molecules in endothelial cells. Bioscience, biotechnology, and biochemistry. 74:1539-1544.
Nivison-Smith, L., J. Rnjak, and A.S. Weiss. 2010. Synthetic human elastin microfibers: Stable cross-linked tropoelastin and cell interactive constructs for tissue engineering applications. Acta Biomaterialia. 6:354-359.
O'Brien, B.J., J.S. Stinson, S.R. Larsen, M.J. Eppihimer, and W.M. Carroll. 2010. A platinum–chromium steel for cardiovascular stents. Biomaterials. 31:3755-3761.
Pasciu, V., A.M. Posadino, A. Cossu, B. Sanna, B. Tadolini, L. Gaspa, A. Marchisio, S. Dessole, G. Capobianco, and G. Pintus. 2010. Akt Downregulation by Flavin Oxidase–Induced ROS Generation Mediates Dose-Dependent Endothelial Cell Damage Elicited by Natural Antioxidants. Toxicological Sciences. 114:101-112.
Patel, N.S., V.S. Mathura, C. Bachmeier, D. Beaulieu-Abdelahad, V. Laporte, O. Weeks, M. Mullan, and D. Paris. 2010. Alzheimer’s β-amyloid peptide blocks vascular endothelial growth factor mediated signaling via direct interaction with VEGFR-2. Journal of Neurochemistry. 112:66-76.
Rajesh, M., P. Mukhopadhyay, G. Haskó, L. Liaudet, K. Mackie, and P. Pacher. 2010. Cannabinoid-1 receptor activation induces reactive oxygen species-dependent and -independent mitogen-activated protein kinase activation and cell death in human coronary artery endothelial cells. British journal of pharmacology. 160:688-700.
Rong, Y., M. Zhang, L. Zhang, X.L. Wang, and Y.H. Shen. 2010. JNK-ATF-2 inhibits thrombomodulin (TM) expression by recruiting histone deacetylase4 (HDAC4) and forming a transcriptional repression complex in the TM promoter. FEBS letters. 584:852-858.
Sheikh-Ali, M., S. Sultan, A.-R. Alamir, M.J. Haas, and A.D. Mooradian. 2010a. Effects of antioxidants on glucose-induced oxidative stress and endoplasmic reticulum stress in endothelial cells. Diabetes research and clinical practice. 87:161-166.
Sheikh-Ali, M., S. Sultan, A.-R. Alamir, M.J. Haas, and A.D. Mooradian. 2010b. Hyperglycemia-induced endoplasmic reticulum stress in endothelial cells. Nutrition. 26:1146-1150.
Villareal, F., P. Taub, A. Maisel, G. Schreiner, A. Murphy, K. Yamazaki, and G. Ceballos. 2010. Methods and compositions for treatment of ischemic conditions and conditions related to mitochondrial function. Patent Application US 20120095063 A1.
Wang, H.-J., W.-Y. Lo, T.-L. Lu, and H. Huang. 2010. (−)-Epigallocatechin-3-gallate decreases thrombin/paclitaxel-induced endothelial tissue factor expression via the inhibition of c-Jun terminal NH2 kinase phosphorylation. Biochemical and biophysical research communications. 391:716-721.
Wu, W. The Role of AMPK and miR-92a in the Shear Stress Regulation of KLF2. PhD Dissertation, UC Riverside.
Zeisberger, S. Zoller, M. Riegel, S. Chen, G. Krenning, M. Harmsen, A. Sachinidis, and A. Zisch. 2010. Optimization of the culturing conditions of human umbilical cord blood-derived endothelial colony-forming cells under xeno-free conditions applying a transcriptomic approach. Genes to Cells, 15:671-687.
Zhang, Y., W. Schulte, D. Pink, K. Phipps, A. Zijlstra, J.D. Lewis, and D.M. Waisman. 2010. Sensitivity of Cancer Cells to Truncated Diphtheria Toxin. PloS one. 5:e10498.
2009
Duffy, G., E. Byrne, T. McFadden, E. Farrell, and F. O’Brien. 2009. IN VITRO VASCULARISATION OF COLLAGEN-GAG SCAFFOLDS USING MESENCHYMAL STEM CELLS. Eu Cells and Materials 18:16.
Hampson, A. 2009. Transcriptional Regulation of Soluble Guanylyl Cyclase. B.Sc. honors thesis, Dublin Institute of Technology.
Hendriks-Balk, M., J. Unen, H. Burt, and J. Butler. 2009. Extracellular S1P disappearance: effect on signalling potency. Source: Balk, M. 2009. Regulation of cardiovascular GPCR signaling. PhD Thesis, U Amsterdam.
Himmelhaus, M., and A. Francois. 2009. In-vitro sensing of biomechanical forces in live cells by a whispering gallery mode biosensor. Biosensors and Bioelectronics. 25:418-427.
Himmelhaus, M., and A. Francois. 2009. Method for sensing a biochemical and/or biomechanical process of a biological material and method for analyzing biological materials. Patent Application US 20110256577 A1.
Huang, C., G. Li, H. Dong, S. Sun, H. Chen, D. Luo, L. Sun, X. Li, Z. Chen, H. Yang, S. Wei and Y. Zhou. 2009. Arg972 insulin receptor substrate-1 inhibits endothelial nitric oxide synthase expression in human endothelial cells by upregulating microRNA-155. Int J Molec Med, http://dx.doi.org/10.3892/ijmm.2015.2192.
Li, R., T. Beebe, J. Cui, M. Rouhanizadeh, L. Ai, P. Wang, M. Gundersen, W. Takabe, and T.K. Hsiai. 2009. Pulsatile shear stress increased mitochondrial membrane potential: Implication of Mn-SOD. Biochemical and biophysical research communications. 388:406-412.
Nichols, C., and B. Yu. 2009. Low Dosage Serotonin 5-HT2A Receptor Agonist To Suppress Inflammation. Patent Application US 20100016280 A1.
Wang, H.-J., H. Huang, Y.-C. Chuang, and H.-C. Huang. 2009. Paclitaxel induces up-regulation of tissue factor in human aortic endothelial cells. International Immunopharmacology. 9:144-147.
Yanagida, K., K. Masago, H. Nakanishi, Y. Kihara, F. Hamano, Y. Tajima, R. Taguchi, T. Shimizu, and S. Ishii. 2009. Identification and Characterization of a Novel Lysophosphatidic Acid Receptor, p2y5/LPA6. Journal of Biological Chemistry. 284:17731-17741.
