Product Sheet CA1787
Description
BACKGROUND Lipoprotein lipase (LPL, EC 3.1.1.34) is a central enzyme in lipid metabolism. It catalyzes hydrolysis of the triglyceride core of triglyceriderich lipoproteins (chylomicrons and VLDLs), delivering free fatty acid (FFA) to peripheral tissues for utilization and storage. It is highly expressed in skeletal muscle and adipose tissue, main TG clearance sites, and plays a key role in substrate partitioning regulation. As a homodimer, it is bound to endothelial heparan sulfate proteoglycans especially in the capillaries of heart muscle, skeletal muscle, and adipose tissue. In this location, many molecules of LPL bind to chylomicrons and VLDLs where they are activated by apolipoprotein CII and lipid hydrolysis ensues. After the release of free fatty acids and monoacylglycerol, chylomicron and VLDL remnants are formed, which also contain LPL released from the hydrolytic site. Although this remnant-bound lipase is largely inactive, the presence of LPL may facilitate receptor-mediated remnant binding, uptake, and processing by tissues. Several studies did demonstrate that Independent of its catalytic activity, LPL can also act as a structural cofactor facilitating cellular uptake of whole lipoprotein particles and selective cholesterol ester uptake.1 It was suggested that the catalytic function of LPL is probably antiatherogenic, while the noncatalytic bridging function may be proatherogenic.
It has also documented premature atherosclerosis, elevated triglyceride-rich lipoproteins, and reduced HDL-C in people with moderately reduced LPL activity caused by LPL mutations. Overexpression of LPL in transgenic mice reduced plasma triglycerides and increased high-density lipoprotein cholesterol (HDL-C) concentration, prevented hypertriglyceridemia after a high-fat or high-sucrose diet, and blunted the lipoprotein disorders associated with diabetes. In addition, overexpression of LPL, specifically in skeletal muscle, was sufficient to impart resistance to diet-induced obesity. A rapid catabolism of HDL apolipoprotein and decreased HDL-C was observed in primates after acute injection of an antibody against LPL. There are many factors that can regulate expression of LPL. It was demonstrated that local contractile activity is required for increasing LPL expression during exercise training and for maintaining a high level of LPL expression in postural muscles.2 However, all-trans retinoic acid (RA) can specifically down-regulate LPL enzyme expression in adipocytes at the posttranscriptional level.3
It has also documented premature atherosclerosis, elevated triglyceride-rich lipoproteins, and reduced HDL-C in people with moderately reduced LPL activity caused by LPL mutations. Overexpression of LPL in transgenic mice reduced plasma triglycerides and increased high-density lipoprotein cholesterol (HDL-C) concentration, prevented hypertriglyceridemia after a high-fat or high-sucrose diet, and blunted the lipoprotein disorders associated with diabetes. In addition, overexpression of LPL, specifically in skeletal muscle, was sufficient to impart resistance to diet-induced obesity. A rapid catabolism of HDL apolipoprotein and decreased HDL-C was observed in primates after acute injection of an antibody against LPL. There are many factors that can regulate expression of LPL. It was demonstrated that local contractile activity is required for increasing LPL expression during exercise training and for maintaining a high level of LPL expression in postural muscles.2 However, all-trans retinoic acid (RA) can specifically down-regulate LPL enzyme expression in adipocytes at the posttranscriptional level.3
REFERENCES
1. Merkel, M. Et al: J. Biol. Chem. 277:7405-11, 2001
2. Hamilton, M.T. et al: Am J Physiol Endocrinol Metab 275: E1016-E1022, 1998
3. Kamei, Y. et al: Biochem. Int. 26:923-34, 1992
2. Hamilton, M.T. et al: Am J Physiol Endocrinol Metab 275: E1016-E1022, 1998
3. Kamei, Y. et al: Biochem. Int. 26:923-34, 1992
Products are for research use only. They are not intended for human, animal, or diagnostic applications.
Details
Cat.No.: | CA1787 |
Antigen: | C-terminus |
Isotype: | Affinity-Purified Rabbit Polyclonal IgG |
Species & predicted species cross- reactivity ( ): | Human, Rat |
Applications & Suggested starting dilutions:* | WB 1:500 to 1:1000 IP n/d IHC (Paraffin) 1:50 to 1:200 ICC n/d FACS n/d |
Predicted Molecular Weight of protein: | 55 kDa |
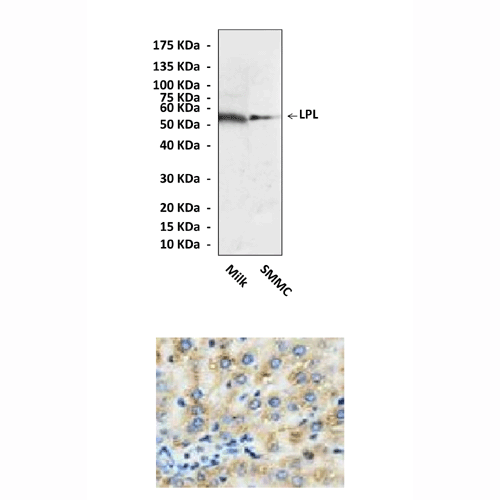
Specificity/Sensitivity: | Reacts specifically with LPL of human & rat origin in immunostaining and Western blot applications. |
Storage: | Store at 4° C for frequent use; at -20° C for at least one year. |
*Optimal working dilutions must be determined by end user.
Resources/Documents
Publications
2010
Wang, X., W. Sun and E., Xu. 2010. The expression and activity of brain lipoprotein lipase is increased after acute cerebral ischemia-reperfusion in rats. Neuropathology, 30:131-139