Choose the specially optimized, ready-to-use EGM that is best suited for your specific cell type and application:
|
Cat# 211-500
Optimized for angiogenesis and other physiological studies (does not contain VEGF).
|
Cat# 213-500
Ready-to-use medium, enriched with growth factors for accelerated growth: VEGF, FBS (2%), bFGF, Heparin, EGF, Hydrocortisone, R3-IGF, Ascorbic acid, Antibiotics.
|
ALSO AVAILABLE:
|
Cat# 213KS-500
All growth supplements packaged as separate vials. Select Cat.# 213K-500 for version with all supplements pre-mixed in one vial.
|
Cat# 212-500
Specifically formulated for Endothelial Cells derived from mid-sized blood vessels (ex. coronary artery endothelial cells).
|
Cat# 111-500
Formulated for Endothelial Cells derived from microvessels. Ranked #1 medium in head-to-head comparison with other leading suppliers, according to Leopold, et al. (PMID: 31541300).
|
Choose the media format that best suits how you will use the media:
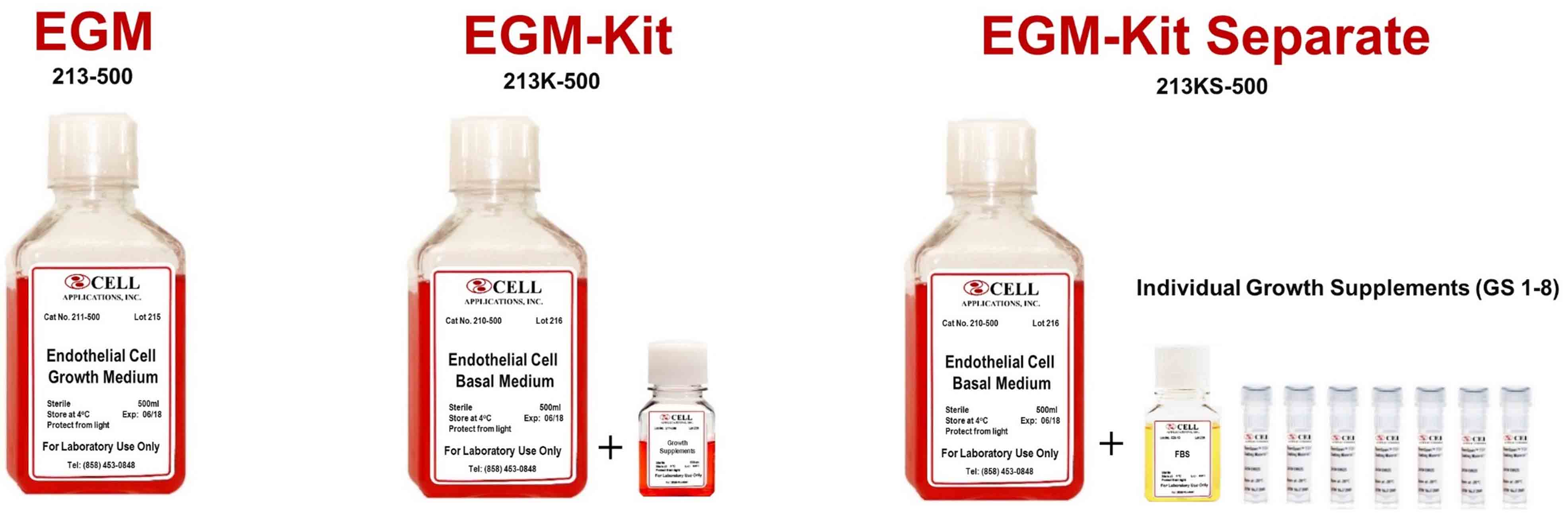
Endothelial Cell Media optimized for primary endothelial cells, microvascular cells, and endothelial cells from mid-sized blood vessels. Endothelial Cell Growth Medium (EGM) is the complete, ready-to-use media that contains all the vitamins, minerals, salts, growth factors, antibiotics, etc. to culture endothelial cells. The medium does not require additional components and can be used upon receipt. The Endothelial Cell Growth Medium Kit (EGM-Kit) format is comprised of the Endothelial Cell Basal Medium (EBM) and Endothelial Cell Growth Supplement (GS) in separate bottles. EBM and GS are mixed together to make the complete EGM. When the EBM and GS are kept apart and properly stored, the shelf life of the EGM Kit is longer than EGM. The EGM-Kit Separate format is similar to the EGM-Kit, except the individual components are vialed and bottled separately. This format is allows for the omission and/or modification of specific supplements.
Specialized media, optimized for the unique requirements of Endothelial Cells. Carefully crafted, quality tested. CAI media are tested for sterility to confirm no bacteria, yeast or fungi contamination, and QC tests for correct pH, osmolality and lack of endotoxins. Bioassays affirm proper culture, growth, plating, karyotype, physiology, morphology, viability, population doublings, surface markers, cryopreservation, differentiation and/or induction.
