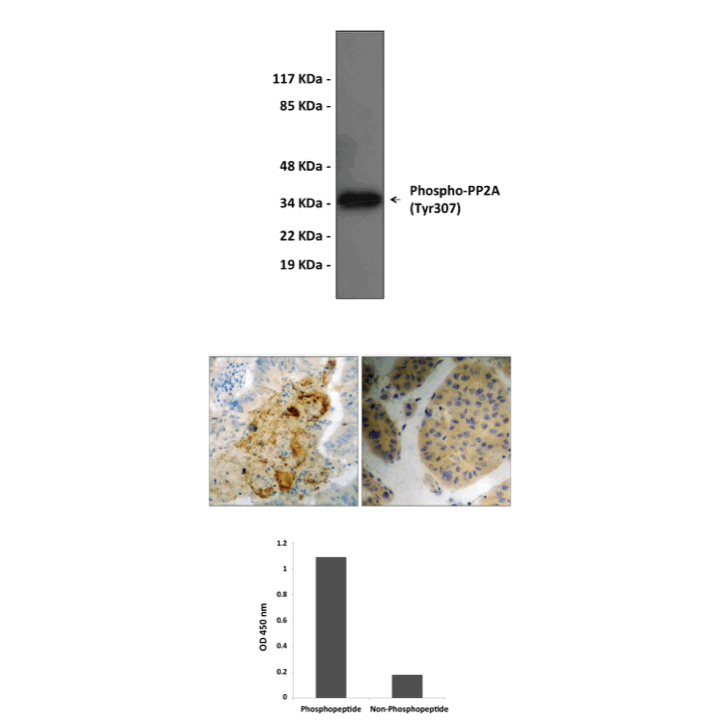
Product Sheet CG1474
Description
BACKGROUND Protein ser/thr phosphatases are a group of enzymes that catalyze the removal of phosphate groups from serine and/or threonine residues by the hydrolysis of phosphoric acid monoesters. They directly oppose the actions of kinases and phosphorylases and therefore play an integral role in many signal transduction pathways. There are two groups of serine/threonine phosphatases; phosphoprotein phosphatases (e.g. PP1, calcineurin), which are sensitive to okadaic acid and metallo-phosphatases (e.g. PP2C), which require a divalent cation, commonly Mg2+, for catalytic activity. Dephosphorylation, depending on the residue that the phosphate group is removed from, can have a stimulatory or inhibitory effect on the target molecule. This makes protein Ser/Thr phosphatases essential for many signal transduction pathways. Protein Ser/Thr phosphatase are regulated by their subcellular localization and by inhibitor proteins, which are subtype-specific.1
PP2A is a ubiquitously expressed protein serine/threonine phosphatase that accounts for a large fraction of phosphatase activity in eukaryotic cells. PP2A is not a single entity but rather a large collection of oligomeric enzymes that contain a common catalytic subunit. The most common forms of PP2A contain an active core dimer composed of the catalytic subunit (C subunit) and a scaffold protein termed the A subunit. The scaffold subunit mediates interaction of the core dimer with a wide variety of regulatory subunits (B subunits) that dictate the functions of individual forms. Multiple families, isoforms, and splice variants of the regulatory subunits allows generation of over 60 different heterotrimeric PP2A holoenzymes. The regulatory subunits typically enhance the formation of stable complexes between PP2A and its substrates. PP2A has the remarkable ability to interact with structurally distinct regulatory subunits and to form complexes with many different substrates due to the inherent flexibility of the scaffold subunit, which is composed of 15 tandem HEAT repeats. The recent solution of the three-dimensional structure of a PP2A holoenzyme has shown that the scaffold subunit undergoes dramatic structural rearrangements following interaction with the catalytic and regulatory subunits. Thus, in addition to diversity generated by the regulatory subunits, flexibility within the PP2A structure provides additional adaptability in substrate recognition. These properties help to account for the large and growing list of phosphoproteins and signaling pathways known to be affected by PP2A.2
Many studies have implicated an important regulatory role for PP2A in a wide variety of cellular functions including metabolism, transcription and translation, ion transport, development, cell growth, and differentiation. In addition to regulatory subunits, PP2A activity is influenced by subunit phosphorylation and carboxymethylation.3 For example, phosphorylation of Tyr307 at PP2A C subunit by Src leads to inhibition of PP2A enzyme activity.4 Thus, both regulatory subunit binding and post-translational modifications are important mechanisms controlling PP2A function. An emerging body of evidence indicates that regulation of PP2A also involves association with other proteins in addition to the core phosphatase subunits. For example, PP2A has been shown to bind to the beta2-adrenergic receptor, casein kinase 2alpha etc. Recently, it was identified a CaMKIV-PP2A signaling complex in which PP2A dephosphorylates the associated CaMKIV and functions as a negative modulator of CaMKIV signaling. Thus, a likely determinant for directing PP2A function is its association with other proteins in multiprotein signaling complexes. It was also demonstrated the existence of p70 S6 kinase-PP2A and PAK-PP2A complexes in rat brain. Thus, the assembly of protein kinase-PP2A signaling modules is a general mechanism for regulation of PP2A action in vivo.5
PP2A is a ubiquitously expressed protein serine/threonine phosphatase that accounts for a large fraction of phosphatase activity in eukaryotic cells. PP2A is not a single entity but rather a large collection of oligomeric enzymes that contain a common catalytic subunit. The most common forms of PP2A contain an active core dimer composed of the catalytic subunit (C subunit) and a scaffold protein termed the A subunit. The scaffold subunit mediates interaction of the core dimer with a wide variety of regulatory subunits (B subunits) that dictate the functions of individual forms. Multiple families, isoforms, and splice variants of the regulatory subunits allows generation of over 60 different heterotrimeric PP2A holoenzymes. The regulatory subunits typically enhance the formation of stable complexes between PP2A and its substrates. PP2A has the remarkable ability to interact with structurally distinct regulatory subunits and to form complexes with many different substrates due to the inherent flexibility of the scaffold subunit, which is composed of 15 tandem HEAT repeats. The recent solution of the three-dimensional structure of a PP2A holoenzyme has shown that the scaffold subunit undergoes dramatic structural rearrangements following interaction with the catalytic and regulatory subunits. Thus, in addition to diversity generated by the regulatory subunits, flexibility within the PP2A structure provides additional adaptability in substrate recognition. These properties help to account for the large and growing list of phosphoproteins and signaling pathways known to be affected by PP2A.2
Many studies have implicated an important regulatory role for PP2A in a wide variety of cellular functions including metabolism, transcription and translation, ion transport, development, cell growth, and differentiation. In addition to regulatory subunits, PP2A activity is influenced by subunit phosphorylation and carboxymethylation.3 For example, phosphorylation of Tyr307 at PP2A C subunit by Src leads to inhibition of PP2A enzyme activity.4 Thus, both regulatory subunit binding and post-translational modifications are important mechanisms controlling PP2A function. An emerging body of evidence indicates that regulation of PP2A also involves association with other proteins in addition to the core phosphatase subunits. For example, PP2A has been shown to bind to the beta2-adrenergic receptor, casein kinase 2alpha etc. Recently, it was identified a CaMKIV-PP2A signaling complex in which PP2A dephosphorylates the associated CaMKIV and functions as a negative modulator of CaMKIV signaling. Thus, a likely determinant for directing PP2A function is its association with other proteins in multiprotein signaling complexes. It was also demonstrated the existence of p70 S6 kinase-PP2A and PAK-PP2A complexes in rat brain. Thus, the assembly of protein kinase-PP2A signaling modules is a general mechanism for regulation of PP2A action in vivo.5
REFERENCES
1. Gallego, M. & Virshup, D.M.: Curr. Opin. Cell Biol. 17:197-202, 2005
2. Zolnierowicz, S.:Biochem. Pharmacol. 60:1225-35, 2000
3. Bryant, J.C. et al: Biochem. J. 339(PtB):241-6, 1999
4. Hu, X. et al: BMC Neurosci. 10:74, 2009
5. Westphal, R.S. et al: J. Biol. Chem. 274:687-92, 1999
2. Zolnierowicz, S.:Biochem. Pharmacol. 60:1225-35, 2000
3. Bryant, J.C. et al: Biochem. J. 339(PtB):241-6, 1999
4. Hu, X. et al: BMC Neurosci. 10:74, 2009
5. Westphal, R.S. et al: J. Biol. Chem. 274:687-92, 1999
Products are for research use only. They are not intended for human, animal, or diagnostic applications.
Details
Contact us regarding this antibody
Cat.No.: | CG1474 |
Antigen: | Range AA292 to 322 |
Isotype: | Rabbit IgG |
Species & predicted species cross- reactivity ( ): | Human, Rat, Mouse |
Applications & Suggested starting dilutions:* | WB 1:500-1:1000 IP n/d IHC 1:50-1:100 ICC n/d FACS n/d ELISA 1:5000 |
Predicted Molecular Weight of protein: | 35 kDa |
Specificity/Sensitivity: | Detects endogenous PP2A (Tyr207) proteins without cross-reactivity with other family members. |
Storage: | Store at -20°C, 4°C for frequent use. Avoid repeated freeze-thaw cycles. |
*Optimal working dilutions must be determined by end user.
Products
| Product | Size | CAT.# | Price | Quantity |
|---|---|---|---|---|
| Rabbit PP2A, Phospho-Tyr307 Antibody: Rabbit PP2A, Phospho-Tyr307 Antibody | Size: 100 ul | CAT.#: CG1474 | Price: $384.00 |