Product Sheet CG1596
Description
BACKGROUND Tau proteins belong to the family of microtubule-associated proteins. They are mainly expressed in neurons where they play an important role in the assembly of tubulin monomers into microtubules to constitute the neuronal microtubules network. Microtubules are involved in maintaining the cell shape and serve as tracks for axonal transport. Tau proteins also establish some links between microtubules and other cytoskeletal elements or proteins. Tau proteins are translated from a single gene located on chromosome 17. Their expression is developmentally regulated by an alternative splicing mechanism and six different isoforms exist in the human adult brain. Tau proteins are the major constituents of intraneuronal and glial fibrillar lesions described in Alzheimer’s disease and numerous neurodegenerative disorders referred to as ‘tauopathies’. Molecular analysis has revealed that an abnormal phosphorylation might be one of the important events in the process leading to their aggregation. Moreover, a specific set of pathological tau proteins exhibiting a typical biochemical pattern, and a different regional and laminar distribution could characterize each of these disorders. Finally, a direct correlation has been established between the progressive involvement of the neocortical areas and the increasing severity of dementia, suggesting that pathological tau proteins are reliable marker of the neurodegenerative process. The discovery of tau gene mutations in frontotemporal dementia with parkinsonism linked to chromosome 17 has reinforced the predominant role attributed to tau proteins in the pathogenesis of neurodegenerative disorders, and underlined the fact that distinct sets of tau isoforms expressed in different neuronal populations could lead to different pathologies.1
Tau has more than 30 potential phosphorylation sites, many of which are putative targets of proline-directed serine/threonine kinases.2 Among these tau kinase candidates, GSK-3b, Cdk5 and PKA have been most implicated. The former two were actually described as tau kinase I and tau kinase II, respectively.3 These two kinases can phosphorylate tau at multiple sites. Tau is hyperphosphorylated at as many as 37 sites in AD brain. Tau phosphorylation at different sites has a different impact on its biological function and on its pathogenic role. A quantitative in vitro study demonstrated that phosphorylation of tau at Ser262, Thr231, and Ser235 inhibits its binding to microtubules by ∼35%, ∼25%, and ∼10%, respectively. In vitro kinetic studies of the binding between hyperphosphorylated tau and normal tau suggest that Ser199/Ser202/Thr205, Thr212, Thr231/Ser235, Ser262/Ser356, and Ser422 are among the critical phosphorylation sites that convert tau to an inhibitory molecule that sequesters normal microtubule-associated proteins from microtubules. Further phosphorylation at Thr231, Ser396, and Ser422 promotes self-aggregation of tau into filaments.4 Similarly, mutation of tau at Ser396 and Ser404 into Glu to mimic phosphoserine converts it to be more fibrillogenic, and a tau construct in which Ser422 is mutated to Glu shows a significantly increased propensity to aggregate. It is obvious that tau phosphorylation at various sites impacts tau activity and aggregation collectively. Recent studies have demonstrated that tau phosphorylation at the proline-rich region, which is located upstream of the microtubule-binding domains, inhibits its microtubule assembly activity moderately and promotes its self-aggregation slightly. Tau phosphorylation at the C-terminal tail region increases its activity and promotes its self-aggregation markedly. Tau phosphorylation at both of these regions plus the microtubule-binding region nearly diminishes its activity and disrupts microtubules.5
Tau has more than 30 potential phosphorylation sites, many of which are putative targets of proline-directed serine/threonine kinases.2 Among these tau kinase candidates, GSK-3b, Cdk5 and PKA have been most implicated. The former two were actually described as tau kinase I and tau kinase II, respectively.3 These two kinases can phosphorylate tau at multiple sites. Tau is hyperphosphorylated at as many as 37 sites in AD brain. Tau phosphorylation at different sites has a different impact on its biological function and on its pathogenic role. A quantitative in vitro study demonstrated that phosphorylation of tau at Ser262, Thr231, and Ser235 inhibits its binding to microtubules by ∼35%, ∼25%, and ∼10%, respectively. In vitro kinetic studies of the binding between hyperphosphorylated tau and normal tau suggest that Ser199/Ser202/Thr205, Thr212, Thr231/Ser235, Ser262/Ser356, and Ser422 are among the critical phosphorylation sites that convert tau to an inhibitory molecule that sequesters normal microtubule-associated proteins from microtubules. Further phosphorylation at Thr231, Ser396, and Ser422 promotes self-aggregation of tau into filaments.4 Similarly, mutation of tau at Ser396 and Ser404 into Glu to mimic phosphoserine converts it to be more fibrillogenic, and a tau construct in which Ser422 is mutated to Glu shows a significantly increased propensity to aggregate. It is obvious that tau phosphorylation at various sites impacts tau activity and aggregation collectively. Recent studies have demonstrated that tau phosphorylation at the proline-rich region, which is located upstream of the microtubule-binding domains, inhibits its microtubule assembly activity moderately and promotes its self-aggregation slightly. Tau phosphorylation at the C-terminal tail region increases its activity and promotes its self-aggregation markedly. Tau phosphorylation at both of these regions plus the microtubule-binding region nearly diminishes its activity and disrupts microtubules.5
REFERENCES
1. Mandelkow, E. et al: Neurobiol. Aging 16:355-62, 1995
2. Liu, F. et al: FEBS Lett. 580:6269-74, 2006
3. Geschwind, D.H. et al: Neuron40:457-60, 2003
4. Gong, C.X. & Iqbal, K.:Curr. Med. Chem. 15:2321-8, 2008
5. Liu, F. et al: Eur. J. Neurosci. 26:3429-36, 2007
2. Liu, F. et al: FEBS Lett. 580:6269-74, 2006
3. Geschwind, D.H. et al: Neuron40:457-60, 2003
4. Gong, C.X. & Iqbal, K.:Curr. Med. Chem. 15:2321-8, 2008
5. Liu, F. et al: Eur. J. Neurosci. 26:3429-36, 2007
Products are for research use only. They are not intended for human, animal, or diagnostic applications.
Details
Cat.No.: | CG1596 |
Antigen: | Range AA570 to 600 |
Isotype: | Rabbit IgG |
Species & predicted species cross- reactivity ( ): | Human, Mouse, Rat |
Applications & Suggested starting dilutions:* | WB 1:500-1:1000 IP n/d IHC n/d ICC n/d FACS n/d ELISA 1:5000 |
Predicted Molecular Weight of protein: | 78 kDa |
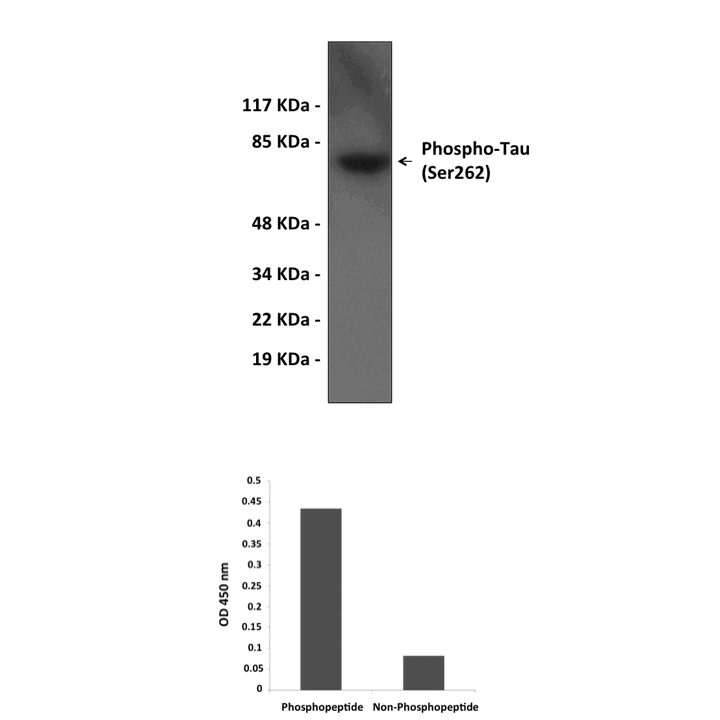
Specificity/Sensitivity: | Detects endogenous phosphorylated Tau proteins (Ser262) without cross-reactivity with other family members. |
Storage: | Store at -20°C, 4°C for frequent use. Avoid repeated freeze-thaw cycles. |
*Optimal working dilutions must be determined by end user.
Products
| Product | Size | CAT.# | Price | Quantity |
|---|---|---|---|---|
| Rabbit Tau, Phospho-Ser262 Antibody: Rabbit Tau, Phospho-Ser262 Antibody | Size: 100 ul | CAT.#: CG1596 | Price: $384.00 |