Product Sheet CG1461
Description
BACKGROUND Phospholipase C (PLC) enzymes, comprising several families (PLCbeta, gamma, delta, epsilon, eta, and zeta), have been established as crucial signaling molecules involved in regulation of a variety of cellular functions. PLC-catalyzed formation of the second messengers, inositol 1,4,5-trisphosphate (IP3) and diacylglycerol, from phosphatidylinositol 4,5-bisphosphate (PIP2), constitutes one of the major cell signaling responses. IP (3) induces a transient increase in intracellular free Ca2+, while DAG directly activates protein kinase C. These second messengers provide a common link from highly specific receptors for hormones, neurotransmitters, antigens, and growth factors to downstream, intracellular targets; thus, they contribute to regulation of biological functions as diverse as cell motility, fertilization, and sensory transduction.1
Of two PLCgamma enzymes, PLCgamma1 is ubiquitously expressed and appears to regulate a multitude of cellular functions in many tissues. It is activated in response to growth factor stimulation; in addition, its function in T-cell responses has been extensively documented. PLCgamma2, in contrast, is most highly expressed in cells of the hematopoietic system and plays a key role in regulation of the immune response. Both PLCgamma enzymes have also been implicated in signaling events underlying aberrant cellular responses. PLCgamma1 is critically involved in the regulation of cancer cell motility while PLCgamma2 has been implicated in deregulation of the immune responses resembling Btk-dependent X-linked agammaglobulinaemia and SLE disease in humans. It has been suggested that, in cancer cells, PLCgamma1 could function as a key, rate-limiting, common component involved in cell motility triggered by several growth factors and integrins.2
The domain organization of PLCgamma enzymes is characterized by the insertion of a highly structured region (PLCgamma-specific array, gammaSA) between the two halves of the TIM-barrel catalytic domain common to all PLCs. The gammaSA comprises a split PH (spPH) domain flanking two tandem SH2 domains and a SH3 domain. A distinct regulatory feature of PLCgamma enzymes is that their activation is linked to an increase in phosphorylation of specific tyrosine residues (most notably within the gammaSA) by receptor and non-receptor tyrosine kinases. Upon stimulation of cells with PDGF and EGF, the SH2 domain of PLC-gamma binds to the autophosphorylated tyrosine residues of growth factor receptors, leading to tyrosine phosphorylation and activation of PLC-gamma.3 In addition, activation of PLC-gamma isozymes may occur secondarily to receptor-mediated activation of phospholipase D and cytosolic phospholipase A2, which results in the production of phosphatidic acid and arachidonic acid, respectively.4 It was also reported that PLC-gamma is regulated additionally by the lipid products of PI 3-kinase. The PH domain of PLC-gamma binds to Pidgins(3,4,5)P3, and is targeted to the membrane in response to growth factor stimulation and leads to activation of PI 3-kinase causes PLC-gamma PH domain-mediated membrane targeting and PLC-gamma activation.5 Furthermore, multiple protein-protein interactions (mainly mediated by SH2 domains) also contribute to activation and have an important role in localizing PLCgamma into protein complexes with different binding partners, depending on cell type and specific cellular compartments. One mode of activation that is specific for the PLCgamma2 isozyme is direct binding to and activation by Rac. The interaction involves the spPH domain, and this activation mechanism does not require tyrosine phosphorylation.6
Of two PLCgamma enzymes, PLCgamma1 is ubiquitously expressed and appears to regulate a multitude of cellular functions in many tissues. It is activated in response to growth factor stimulation; in addition, its function in T-cell responses has been extensively documented. PLCgamma2, in contrast, is most highly expressed in cells of the hematopoietic system and plays a key role in regulation of the immune response. Both PLCgamma enzymes have also been implicated in signaling events underlying aberrant cellular responses. PLCgamma1 is critically involved in the regulation of cancer cell motility while PLCgamma2 has been implicated in deregulation of the immune responses resembling Btk-dependent X-linked agammaglobulinaemia and SLE disease in humans. It has been suggested that, in cancer cells, PLCgamma1 could function as a key, rate-limiting, common component involved in cell motility triggered by several growth factors and integrins.2
The domain organization of PLCgamma enzymes is characterized by the insertion of a highly structured region (PLCgamma-specific array, gammaSA) between the two halves of the TIM-barrel catalytic domain common to all PLCs. The gammaSA comprises a split PH (spPH) domain flanking two tandem SH2 domains and a SH3 domain. A distinct regulatory feature of PLCgamma enzymes is that their activation is linked to an increase in phosphorylation of specific tyrosine residues (most notably within the gammaSA) by receptor and non-receptor tyrosine kinases. Upon stimulation of cells with PDGF and EGF, the SH2 domain of PLC-gamma binds to the autophosphorylated tyrosine residues of growth factor receptors, leading to tyrosine phosphorylation and activation of PLC-gamma.3 In addition, activation of PLC-gamma isozymes may occur secondarily to receptor-mediated activation of phospholipase D and cytosolic phospholipase A2, which results in the production of phosphatidic acid and arachidonic acid, respectively.4 It was also reported that PLC-gamma is regulated additionally by the lipid products of PI 3-kinase. The PH domain of PLC-gamma binds to Pidgins(3,4,5)P3, and is targeted to the membrane in response to growth factor stimulation and leads to activation of PI 3-kinase causes PLC-gamma PH domain-mediated membrane targeting and PLC-gamma activation.5 Furthermore, multiple protein-protein interactions (mainly mediated by SH2 domains) also contribute to activation and have an important role in localizing PLCgamma into protein complexes with different binding partners, depending on cell type and specific cellular compartments. One mode of activation that is specific for the PLCgamma2 isozyme is direct binding to and activation by Rac. The interaction involves the spPH domain, and this activation mechanism does not require tyrosine phosphorylation.6
REFERENCES
1. Kim, M.G. et al: Exp. Mol. Med. 32:101-9, 2000
2. Patterson, R.L. et al: Trends in Biochem. Chem. 30:688-97, 2005
3. Nishibe, S. & Carpenter, G.: Semin Cancer Biol. 1:285-92, 1990
4. Sekiya, F. et al:Chem. Phys. Lipids 98:3-11, 1998
5. Falasca, M. et al: EMBO J. 17:414-22, 1998
6. Carpenter,G. & Ji, Q.: Exp. Cell Res. 253:15-24, 1999
2. Patterson, R.L. et al: Trends in Biochem. Chem. 30:688-97, 2005
3. Nishibe, S. & Carpenter, G.: Semin Cancer Biol. 1:285-92, 1990
4. Sekiya, F. et al:Chem. Phys. Lipids 98:3-11, 1998
5. Falasca, M. et al: EMBO J. 17:414-22, 1998
6. Carpenter,G. & Ji, Q.: Exp. Cell Res. 253:15-24, 1999
Products are for research use only. They are not intended for human, animal, or diagnostic applications.
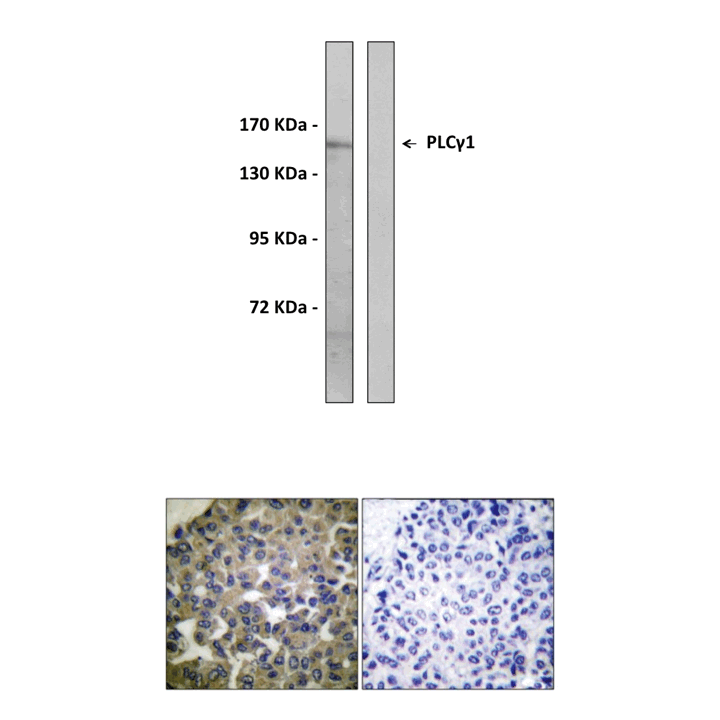
(Click to Enlarge) Top: Immunoblotting analysis of extracts from HepG2, using Anti-PLCG1 antibody. The lane on the left was treated with the Anti-PLCG1 antibody. The lane on the right (negative control) was treated with both Anti-PLCG1 antibody and the synthesized immunogen peptide. Bottom: Immunohistochemistry analysis of paraffin-embedded human breast carcinoma tissue using Anti-PLCG1 antibody. Cells on the left were treated with the Anti-PLCG1 antibody. Cells on the right (negative control) were treated with both Anti-PLCG1 antibody and the synthesized immunogen peptide.
Details
Cat.No.: | CG1461 |
Antigen: | Synthesized peptide derived from human PLCG1. |
Isotype: | Rabbit IgG |
Species & predicted species cross- reactivity ( ): | Human, Rat, Mouse |
Applications & Suggested starting dilutions:* | WB 1:500-1:1000 IP n/d IHC 1:50-1:100 ICC n/d FACS n/d |
Predicted Molecular Weight of protein: | 148 kDa |
Specificity/Sensitivity: | Detects endogenous PLCγ1 proteins without cross-reactivity with other family members. |
Storage: | Store at -20°C, 4°C for frequent use. Avoid repeated freeze-thaw cycles. |
*Optimal working dilutions must be determined by end user.
Products
| Product | Size | CAT.# | Price | Quantity |
|---|---|---|---|---|
| Rabbit Phospholipase C Gamma 1 Antibody: Rabbit Phospholipase C gamma 1 Antibody | Size: 100 ul | CAT.#: CG1461 | Price: $384.00 |
Resources/Documents
Publications
2011
Ehrlich, L., G. Medina, and C. Carter. 2011. ESCRT Machinery Potentiates HIV-1 Utilization of the PI (4, 5) P 2-PLC-IP3R-Ca 2+ Signaling Cascade. J Mol Biol, 413:347-358.
