Product Sheet CP10272
Description
BACKGROUND Smads, the only substrates for type I receptor kinases, were first identified as the products of the Drosophila Mad and C. elegans Sma genes. The human genome encodes eight Smad family members (Mad-homologues (MADH)). MADH2, MADH4 and MADH7 map to chromosome 18q21-22, a tumour suppressor locus; MADH3 and MADH6 map to chromosome 15q21-22, and MADH5, MADH1 and MADH8 to chromosomes 15q31, 4 and 13, respectively. Smads are ubiquitously expressed throughout development and in all adult tissues, and many of them (Smad2, Smad4, Smad5, Smad6 and Smad8) are produced from alternatively spliced mRNAs. Functionally, Smads fall into three subfamilies: receptor-activated Smads (R-Smads: Smad1, Smad2, Smad3, Smad5, Smad8), which become phosphorylated by the type I receptors; common mediator Smads (Co-Smads: Smad4), which oligomerise with activated R-Smads; and inhibitory Smads (I-Smads: Smad6 and Smad7), which are induced by TGF-beta family members. The latter exert a negative feedback effect by competing with R-Smads for receptor interaction and by marking the receptors for degradation. Smads have two conserved domains, the N-terminal Mad homology 1 (MH1) and C-terminal Mad homology 2 (MH2) domains. The MH1 domain is highly conserved among R-Smads and Co-Smads; however, the N-terminal parts of I-Smads have only weak sequence similarity to MH1 domains. The MH1 domain regulates nuclear import and transcription by binding to DNA and interacting with nuclear proteins.1
Smad proteins transduce signals from transforming growth factor-beta (TGF-beta) superfamily ligands that regulate cell proliferation, differentiation and death through activation of receptor serine/threonine kinases. It has been demonstrated that accessory/scaffolding proteins interact with the type I and II receptors and/or the Smads. One example is SARA (Smad anchor for receptor activation), a cytoplasmic protein that specifically interacts with non-activated Smad2 and the receptor complex, thus forming a bridge between the receptor and Smad2 and assisting in the specific phosphorylation of Smad2 by the type I receptor. The mechanism that organises such Smad signalling centres and its links to receptor endocytosis, degradation and signalling crosstalk could provide cell-context specificity, allowing differential regulation of the basic Smad pathway.2
Phosphorylation of the C-terminal serine residues in R-Smads by type I receptor kinases is a crucial step in TGF-beta family signaling. The two most C-terminal serine residues become phosphorylated and, together with a third, non-phosphorylated serine residue, form an evolutionarily conserved SSXS motif in all R-Smads. TGF-beta and activin receptors phosphorylate Smad2 and Smad3, and BMP receptors phosphorylate Smad1, Smad5 and Smad8. Other kinases might also phosphorylate the Smads, which include MAPK, CaMK II and PKC.3 Unphosphorylated Smad proteins exist primarily as monomers, and upon phosphorylation, R-Smads form homo-oligomers, which quickly convert to hetero-oligomers containing the Co-Smad, Smad4 and are imported to the nucleus. Nuclear Smad oligomers bind to DNA and associate with transcription factors to regulate expression of target genes. Alternatively, nuclear R-Smads associate with ubiquitin ligases and promote degradation of transcriptional repressors, thus facilitating target gene regulation by TGF-beta. Smads themselves can also become ubiquitinated and are degraded by proteasomes. Finally, the inhibitory Smads (I-Smads) block phosphorylation of R-Smads by the receptors and promote ubiquitination and degradation of receptor complexes, thus inhibiting signaling.4
Smad proteins transduce signals from transforming growth factor-beta (TGF-beta) superfamily ligands that regulate cell proliferation, differentiation and death through activation of receptor serine/threonine kinases. It has been demonstrated that accessory/scaffolding proteins interact with the type I and II receptors and/or the Smads. One example is SARA (Smad anchor for receptor activation), a cytoplasmic protein that specifically interacts with non-activated Smad2 and the receptor complex, thus forming a bridge between the receptor and Smad2 and assisting in the specific phosphorylation of Smad2 by the type I receptor. The mechanism that organises such Smad signalling centres and its links to receptor endocytosis, degradation and signalling crosstalk could provide cell-context specificity, allowing differential regulation of the basic Smad pathway.2
Phosphorylation of the C-terminal serine residues in R-Smads by type I receptor kinases is a crucial step in TGF-beta family signaling. The two most C-terminal serine residues become phosphorylated and, together with a third, non-phosphorylated serine residue, form an evolutionarily conserved SSXS motif in all R-Smads. TGF-beta and activin receptors phosphorylate Smad2 and Smad3, and BMP receptors phosphorylate Smad1, Smad5 and Smad8. Other kinases might also phosphorylate the Smads, which include MAPK, CaMK II and PKC.3 Unphosphorylated Smad proteins exist primarily as monomers, and upon phosphorylation, R-Smads form homo-oligomers, which quickly convert to hetero-oligomers containing the Co-Smad, Smad4 and are imported to the nucleus. Nuclear Smad oligomers bind to DNA and associate with transcription factors to regulate expression of target genes. Alternatively, nuclear R-Smads associate with ubiquitin ligases and promote degradation of transcriptional repressors, thus facilitating target gene regulation by TGF-beta. Smads themselves can also become ubiquitinated and are degraded by proteasomes. Finally, the inhibitory Smads (I-Smads) block phosphorylation of R-Smads by the receptors and promote ubiquitination and degradation of receptor complexes, thus inhibiting signaling.4
REFERENCES
1. Feng, X.H. & Derynck, R.: Ann. Rev. Cell Dev. Biol. 21:659-93, 2005
2. Dijke, P.T. & Hill, C.S. : Trends Biochem. Sci. 29:265-73, 2004
3. Massagué, J. et al: Gene Dev. 19:2783-10, 2005
4. Miyazawa, K. et al: Gene. Cell.7:1191-1204, 2002
2. Dijke, P.T. & Hill, C.S. : Trends Biochem. Sci. 29:265-73, 2004
3. Massagué, J. et al: Gene Dev. 19:2783-10, 2005
4. Miyazawa, K. et al: Gene. Cell.7:1191-1204, 2002
Products are for research use only. They are not intended for human, animal, or diagnostic applications.
Details
Cat.No.: | CP10272 |
Antigen: | Purified recombinant human Smad3 fragments expressed in E. coli. |
Isotype: | Mouse IgG1 |
Species & predicted species cross- reactivity ( ): | Human, Mouse, Rat |
Applications & Suggested starting dilutions:* | WB 1:1000 IP n/d IHC n/d ICC 1:50 - 1:200 FACS n/d |
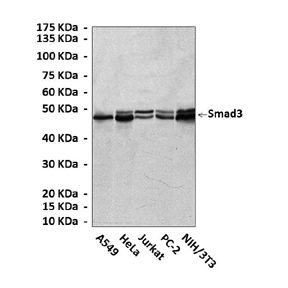
Predicted Molecular Weight of protein: | 48 kDa |
Specificity/Sensitivity: | Detects Smad3 proteins without cross-reactivity with other related proteins. |
Storage: | Store at -20°C, 4°C for frequent use. Avoid repeated freeze-thaw cycles. |
*Optimal working dilutions must be determined by end user.
Products
| Product | Size | CAT.# | Price | Quantity |
|---|---|---|---|---|
| Mouse Smad3 Antibody: Mouse Smad3 Antibody | Size: 100 ul | CAT.#: CP10272 | Price: $413.00 |