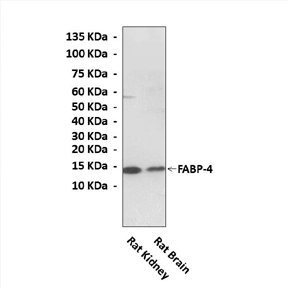
Description
BACKGROUND Fatty acid-binding proteins (FABPs) are members of the superfamily of lipid-binding proteins (LBP). So far 9 different FABPs, with tissue-specific distribution, have been identified: L (liver), I (intestinal), H (muscle and heart), A (adipocyte), E (epidermal), Il (ileal), B (brain), M (myelin) and T (testis). The primary role of all the FABP family members is regulation of fatty acid uptake and intracellular transport. The structure of all FABPs is similar – the basic motif characterizing these proteins is beta-barrel, and a single ligand (e.g. a fatty acid, cholesterol, or retinoid) is bound in its internal water-filled cavity. Despite the wide variance in the protein sequence, the gene structure is identical. The FABP genes consist of 4 exons and 3 introns and a few of them are located in the same chromosomal region. For example, A-FABP, E-FABP and M-FABP create a gene cluster.1 Physiological roles of these proteins are not only involved in FA transport, but also in regulation of cell growth and differentiation, cellular signaling, gene transcription and cytoprotection.2 Because of their physiological properties some FABP genes were tested in order to identify mutations altering lipid metabolism and relating with other diseases.
Adipose fatty acid binding protein 4 (FABP-4, also called Ap2 or A-FABP) is a key mediator of intracellular transport and metabolism of fatty acids in adipose tissues. FABP4 belongs to the intracellular lipid binding protein (iLBP) family. Both iLBPs and lipocalins, such as retinol binding protein (RBP4) and lipocalin-2, which are also linked to insulin resistance, are small, extracellular proteins sharing several common molecular recognition properties. These properties include the binding of small, principally hydrophobic molecules, which makes them ideal transporters of lipid molecules. FABP4 binds fatty acids with high affinity and transports them to various compartments in the cell. Knock-out mice studies suggest that FABP4 may modulate fat mass and lipolysis through its action on hormone-sensitive lipase (HSL), perilipin, and LPL, among others. In addition, when in complex with fatty acids, FABP-4 interacts with and modulates the activity of peroxisome proliferator-activated receptor γ. Genetic studies in mice clearly indicated that deregulation of FABP4 function may lead to the development of severe diseases such as diabetes II type and atherosclerosis. FABP-4 has been proposed as a biochemical mediator of insulin resistance in obese patients, and patient plasma levels are predictors of metabolic syndrome development. Moreover, FABP4 has been suggested to be a bridge between inflammation and other pathways related to the metabolic syndrome.3
Adipose fatty acid binding protein 4 (FABP-4, also called Ap2 or A-FABP) is a key mediator of intracellular transport and metabolism of fatty acids in adipose tissues. FABP4 belongs to the intracellular lipid binding protein (iLBP) family. Both iLBPs and lipocalins, such as retinol binding protein (RBP4) and lipocalin-2, which are also linked to insulin resistance, are small, extracellular proteins sharing several common molecular recognition properties. These properties include the binding of small, principally hydrophobic molecules, which makes them ideal transporters of lipid molecules. FABP4 binds fatty acids with high affinity and transports them to various compartments in the cell. Knock-out mice studies suggest that FABP4 may modulate fat mass and lipolysis through its action on hormone-sensitive lipase (HSL), perilipin, and LPL, among others. In addition, when in complex with fatty acids, FABP-4 interacts with and modulates the activity of peroxisome proliferator-activated receptor γ. Genetic studies in mice clearly indicated that deregulation of FABP4 function may lead to the development of severe diseases such as diabetes II type and atherosclerosis. FABP-4 has been proposed as a biochemical mediator of insulin resistance in obese patients, and patient plasma levels are predictors of metabolic syndrome development. Moreover, FABP4 has been suggested to be a bridge between inflammation and other pathways related to the metabolic syndrome.3
REFERENCES
1. Merkel, M. Et al: J. Biol. Chem. 277:7405-11, 2001
2. Hamilton, M.T. et al: Am J Physiol Endocrinol Metab 275: E1016-E1022, 1998
3. Cabre, A. et al: J. Lipid Res. 49:1746-51, 2008
2. Hamilton, M.T. et al: Am J Physiol Endocrinol Metab 275: E1016-E1022, 1998
3. Cabre, A. et al: J. Lipid Res. 49:1746-51, 2008
Products are for research use only. They are not intended for human, animal, or diagnostic applications.
Details
|
Cat.No.:
|
CA1209
|
|
Antigen:
|
Short peptide from human FABP-4 sequence.
|
|
Isotype:
|
Rabbit IgG
|
|
Species & predicted
species cross-
reactivity ( ):
|
Human, Rat
|
|
Applications &
Suggested starting
dilutions:*
|
WB 1:1000
IP n/d
IHC 1:50 - 1:200
ICC n/d
FACS n/d
|
|
Predicted Molecular
Weight of protein:
|
14 kDa
|
|
Specificity/Sensitivity:
|
Detects endogenous levels of FABP-4 proteins without cross-reactivity with other related proteins.
|
|
Storage:
|
Store at -20°C, 4°C for frequent use. Avoid repeated freeze-thaw cycles.
|
*Optimal working dilutions must be determined by end user.