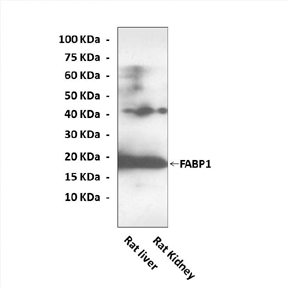
Description
BACKGROUND Fatty acid-binding proteins (FABPs) are members of the superfamily of lipid-binding proteins (LBP). So far 9 different FABPs, with tissue-specific distribution, have been identified: L (liver), I (intestinal), H (muscle and heart), A (adipocyte), E (epidermal), Il (ileal), B (brain), M (myelin) and T (testis). The primary role of all the FABP family members is regulation of fatty acid uptake and intracellular transport. The structure of all FABPs is similar – the basic motif characterizing these proteins is beta-barrel, and a single ligand (e.g. a fatty acid, cholesterol, or retinoid) is bound in its internal water-filled cavity. Despite the wide variance in the protein sequence, the gene structure is identical. The FABP genes consist of 4 exons and 3 introns and a few of them are located in the same chromosomal region. For example, A-FABP, E-FABP and M-FABP create a gene cluster.1 Physiological roles of these proteins are not only involved in FA transport, but also in regulation of cell growth and differentiation, cellular signaling, gene transcription and cytoprotection.2 Because of their physiological properties some FABP genes were tested in order to identify mutations altering lipid metabolism and relating with other diseases.
Liver fatty acid-binding protein (L-FABP or FABP1) is a 14kDa cytoplasmic protein exhibiting a strong affinity for long-chain fatty acids (LCFA). It is highly expressed in liver, where it represents more than 5% of cytosolic proteins. The putative functions assigned to L-FABP/FABP-1 include the desorption of LCFAs from the plasma membrane to the cytoplasm, the promotion of intracellular fatty acid (FA) diffusion, the targeting of FAs to different metabolic pathways and protection against the cytotoxic effects of free FA. Roles in signal transduction pathways and gene regulation have also been reported. The involvement of L-FABP/FABP-1 in the metabolic adaptation to changes in lipid content of the diet can therefore be envisaged in tissues in which it is expressed at substantial levels (i.e. small intestine and liver).3 The transcription rate of the L-FABP/FABP-1 gene is tightly regulated and induced by both fibrate hypolipidemic drugs and LCFA through a peroxisome proliferator-activated receptor (PPAR)-responsive element located in the proximal part of the promoter. It has further been demonstrated that L-FABP may mediate its own expression by enhancing LCFA and LCFA-CoA transport into nuclei to facilitate transcriptional activity of PPAR-alpha. Moreover, L-FABP/FABP-1 regulates PPAR-alpha transcriptional activity in hepatocytes through direct interaction with PPAR-alpha.4 Furthermore L-FABP/FABP-1 expression can also affect the metabolic fate of LCFA. In the mouse, targeted deletion of the L-FABP/FABP-1 gene resulted in a reduced rate of LCFA uptake by the liver under conditions of high lipid supply (e.g. intravenous LCFA bolus or prolonged fasting). Fasted L-FABP/FABP-1 -null mice were characterized by lower hepatic TG synthesis and fatty acid oxidation than wild-type controls. This phenotype is likely caused by the limitation of cellular LCFA availability for further metabolic use (i.e. esterification and oxidation) under high fatty acid load.
Liver fatty acid-binding protein (L-FABP or FABP1) is a 14kDa cytoplasmic protein exhibiting a strong affinity for long-chain fatty acids (LCFA). It is highly expressed in liver, where it represents more than 5% of cytosolic proteins. The putative functions assigned to L-FABP/FABP-1 include the desorption of LCFAs from the plasma membrane to the cytoplasm, the promotion of intracellular fatty acid (FA) diffusion, the targeting of FAs to different metabolic pathways and protection against the cytotoxic effects of free FA. Roles in signal transduction pathways and gene regulation have also been reported. The involvement of L-FABP/FABP-1 in the metabolic adaptation to changes in lipid content of the diet can therefore be envisaged in tissues in which it is expressed at substantial levels (i.e. small intestine and liver).3 The transcription rate of the L-FABP/FABP-1 gene is tightly regulated and induced by both fibrate hypolipidemic drugs and LCFA through a peroxisome proliferator-activated receptor (PPAR)-responsive element located in the proximal part of the promoter. It has further been demonstrated that L-FABP may mediate its own expression by enhancing LCFA and LCFA-CoA transport into nuclei to facilitate transcriptional activity of PPAR-alpha. Moreover, L-FABP/FABP-1 regulates PPAR-alpha transcriptional activity in hepatocytes through direct interaction with PPAR-alpha.4 Furthermore L-FABP/FABP-1 expression can also affect the metabolic fate of LCFA. In the mouse, targeted deletion of the L-FABP/FABP-1 gene resulted in a reduced rate of LCFA uptake by the liver under conditions of high lipid supply (e.g. intravenous LCFA bolus or prolonged fasting). Fasted L-FABP/FABP-1 -null mice were characterized by lower hepatic TG synthesis and fatty acid oxidation than wild-type controls. This phenotype is likely caused by the limitation of cellular LCFA availability for further metabolic use (i.e. esterification and oxidation) under high fatty acid load.
REFERENCES
1. Merkel, M. Et al: J. Biol. Chem. 277:7405-11, 2001
2. Hamilton, M.T. et al: Am J Physiol Endocrinol Metab 275: E1016-E1022, 1998
3. Poirier, H. et al: Biochem. J. 355:481-8, 2001
4. Hostetler, H.A. et al: J. Lipid. Res. 50:1663-75, 2009
2. Hamilton, M.T. et al: Am J Physiol Endocrinol Metab 275: E1016-E1022, 1998
3. Poirier, H. et al: Biochem. J. 355:481-8, 2001
4. Hostetler, H.A. et al: J. Lipid. Res. 50:1663-75, 2009
Products are for research use only. They are not intended for human, animal, or diagnostic applications.
Details
|
Cat.No.:
|
CA1229
|
|
Antigen:
|
Short peptide from human FABP1 sequence.
|
|
Isotype:
|
Rabbit IgG
|
|
Species & predicted
species cross-
reactivity ( ):
|
Human, Mouse, Rat
|
|
Applications &
Suggested starting
dilutions:*
|
WB 1:1000
IP n/d
IHC n/d
ICC n/d
FACS n/d
|
|
Predicted Molecular
Weight of protein:
|
17 kDa
|
|
Specificity/Sensitivity:
|
Detects endogenous levels of FABP1 proteins without cross-reactivity with other related proteins.
|
|
Storage:
|
Store at -20°C, 4°C for frequent use. Avoid repeated freeze-thaw cycles.
|
*Optimal working dilutions must be determined by end user.