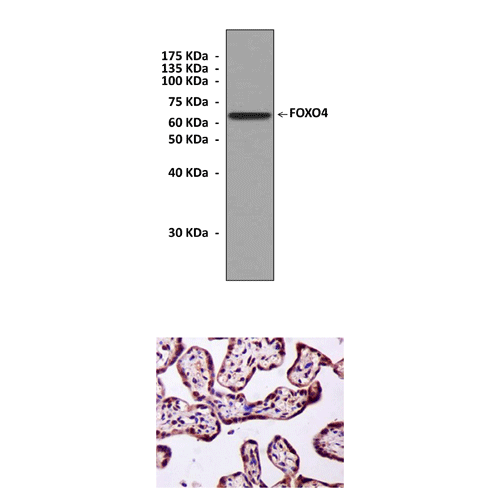
Description
BACKGROUND FOXO4, together with FOXO1, FOXO3 and FOXO6, belongs to a subset of the Forkhead family of transcription factors. Like other Forkhead box O (FOXO) transcription factors, FOXO4 is able to modulate the expression of genes involved in oxidative stress dependent apoptosis, cell cycle arrest, DNA damage repair and other cellular functions. It was shown that FOXO4 proteins reduce oxidative stress by directly increasing mRNA and protein levels of manganese superoxide dismutase (MnSOD) and catalase. Furthermore, FOXO4 can up-regulate the cyclin-dependent kinase inhibitor gene, p27Kip1, causing cell cycle arrest, DNA repair, and is thus tumor suppressive. When ineffective DNA repair or excessive damage ensues, cell death is triggered. Thus, FOXO4 may function as a switch between cell death and survival under oxidative stress stimulation.1 In addition, FOXO4 was shown to play a role in regulating lipid metabolism. It was reported that in an overexpression cellular model, FoxO4 inhibits the late steps of cholesterol biosynthesis, leading to the accumulation of 24,25 dihydrolanosterol (DHL), which impacts triacylglycerol (TAG) accumulation and basal glucose uptake, likely through the stimulation of the liver X receptor α (LXRα).2 Moreover, FoxO4 is an endogenous inhibitor of NF-kappaB and FoxO4 plays important role in the regulation of NF-kappaB-mediated mucosal immunity.3
The FOXO4 transcription factor is regulated in cellular shuttling and DNA binding of target genes by multiple mechanisms. Normally, AKT negatively regulates all FOXO protein activity by direct phosphorylation, causing their nuclear exclusion. However, oxidative stress causes nuclear localization of FOXO4 through Mdm2-dependent mono-ubiquitination and resultant transcriptional activation increase through JNK-mediated phosphorylation at residues 471 and 451. Furthermore, the longevity protein, SIRT1, deacetylates FOXO proteins and modulates their transcriptional activity in response to stress stimuli. In addition, O-GlcNAcylation is an abundant and dynamic post-translational modification (PTM) on serine and threonine residues of nuclear and cytoplasmic proteins. O-GlcNAc Transferase (OGT) and Nuclear Cytoplasmic O-GlcNAcase and Acetyltransferase (NCOAT) catalyze this process of adding and removing the O-GlcNAc groups respectively. O-GlcNAcylation plays a critical role in protein turnover, cell cycle progression, transcription, stress response and other cellular functions. The regulation of FOXO4 in response to oxidative stress involves many PTMs that include, phosphorylation, acetylation and ubiquitination. It was shown that FOXO4 is also O-GlcNAcylated and that this modification is increased upon acute oxidative stress treatment. Furthermore, O-GlcNAc modification causes increased transcriptional activity of FOXO4 in stress-related genes.4 Acetylated Foxo4 promotes the expression of a pro-apoptosis gene Bcl2l11 (also known as Bim) and leads podocyte apoptosis.5
The FOXO4 transcription factor is regulated in cellular shuttling and DNA binding of target genes by multiple mechanisms. Normally, AKT negatively regulates all FOXO protein activity by direct phosphorylation, causing their nuclear exclusion. However, oxidative stress causes nuclear localization of FOXO4 through Mdm2-dependent mono-ubiquitination and resultant transcriptional activation increase through JNK-mediated phosphorylation at residues 471 and 451. Furthermore, the longevity protein, SIRT1, deacetylates FOXO proteins and modulates their transcriptional activity in response to stress stimuli. In addition, O-GlcNAcylation is an abundant and dynamic post-translational modification (PTM) on serine and threonine residues of nuclear and cytoplasmic proteins. O-GlcNAc Transferase (OGT) and Nuclear Cytoplasmic O-GlcNAcase and Acetyltransferase (NCOAT) catalyze this process of adding and removing the O-GlcNAc groups respectively. O-GlcNAcylation plays a critical role in protein turnover, cell cycle progression, transcription, stress response and other cellular functions. The regulation of FOXO4 in response to oxidative stress involves many PTMs that include, phosphorylation, acetylation and ubiquitination. It was shown that FOXO4 is also O-GlcNAcylated and that this modification is increased upon acute oxidative stress treatment. Furthermore, O-GlcNAc modification causes increased transcriptional activity of FOXO4 in stress-related genes.4 Acetylated Foxo4 promotes the expression of a pro-apoptosis gene Bcl2l11 (also known as Bim) and leads podocyte apoptosis.5
REFERENCES
1. Storz, P.: Antioxid. Redox. Signal. 14:593-605, 2011
2. Zhu, J. et al: J. Lipid Res. 51:1312–24, 2010
3. Zhou, W. et al: Gastroenterol. 137:1403-14, 2009
4. Ho, S.R. et al: FEBS Lett. 584:49–54, 2010
5. Chuang, P.Y. et al: PLoS One 6:e23566, 2011
2. Zhu, J. et al: J. Lipid Res. 51:1312–24, 2010
3. Zhou, W. et al: Gastroenterol. 137:1403-14, 2009
4. Ho, S.R. et al: FEBS Lett. 584:49–54, 2010
5. Chuang, P.Y. et al: PLoS One 6:e23566, 2011
Products are for research use only. They are not intended for human, animal, or diagnostic applications.
Details
|
Cat.No.:
|
CG1786
|
|
Antigen:
|
Raised against purified recombinant human FOXO4 proteins expressed in E. coli.
|
|
Isotype:
|
Rabbit IgG
|
|
Species & predicted
species cross-
reactivity ( ):
|
Human
|
|
Applications &
Suggested starting
dilutions:*
|
WB 1:1,000 - 1:10,000
IP n/d
IHC 1:50 - 1:200
ICC n/d
FACS n/d
|
|
Predicted Molecular
Weight of protein:
|
65 kDa
|
|
Specificity/Sensitivity:
|
Detects FOXO4 proteins without cross-reactivity with other family members.
|
|
Storage:
|
Store at -20°C, 4°C for frequent use. Avoid repeated freeze-thaw cycles.
|
*Optimal working dilutions must be determined by end user.