Product Sheet CP10201
Description
BACKGROUND Pirh2 (p53-inducible protein with RING-H2 domain), also known as androgen receptor N-terminal-interacting protein (ARNIP), is a RING-H2 type E3 ubiquitin ligase. It has several isoforms with different expression profiles. Pirh2 was identified as a p53-inducible and -interacting protein which promotes ubiquitylation and degradation of p53. Moreover, Pirh2 is itself a p53-inducible gene product, and this dependence creates an autoregulatory feedback loop in which both the activity of p53 and the expression of Pirh2 are tightly regulated. As well-known, another ubiquitin E3 ligase MDM2 is also a critical p53 regulator. It was shown that SCYL1-BP1 binds to ubiquitin E3 ligase MDM2 and promotes MDM2 self-ubiquitination and results in a reduction of MDM2 protein level. Interestingly, SCYL1-BP1 can be ubiquitinated and degraded by Pirh2 but not by MDM2, which suggested that there is a regulatory network for p53 activity and stability.1 It was also shown that Pirh2 interacts with various molecules including histone acetylase TIP60 (Tat-interactive protein of 60 kDa), measles virus phosphoprotein, N-terminal kinase-like protein-binding protein 1 (NTKL-BP1), and calmodulin. In addition, histone deacetylase 1 (HDAC1) and the epsilon-subunit of coatomer complex (epsilon-COP) were found to be ubiquitylated and subsequently degraded by Pirh2. It was also shown that Pirh2 promotes ubiquitin-dependent degradation of the CDK inhibitor p27Kip1 at the G1/S phase of the cell cycle. These studies suggest that Pirh2 is involved in various cellular events by ubiquitylating and/or interacting with these molecules. Secretory proteins are synthesized with an N-terminal hydrophobic signal sequence. As the signal sequence emerges from the ribosome, it is recognized by the signal recognition particle (SRP) and subsequently targeting of the ribosome-nascent chain complex to the endoplasmic reticulum (ER) membrane occurs. At the membrane, SRP contacts the SRP receptor (SR), a heterodimer composed of SRα and SRbeta. Contact between SRP and SRalpha leads to the transfer of the nascent chain from SRP into the lumen of the ER through the translocation channel formed by the Sec61p complex. It was shown that SRbeta is a novel Pirh2-interacting protein and Pirh2 promoted ubiquitylation of SRbeta without affecting the stability of SRbeta. Thus Pirh2 may control the synthesis of secretory protein by the functional regulation of SRbeta via ubiquitylation.2
As a short-lived protein, Pirh2 is also a target for RING domain-dependent proteasomal degradation. Binding of Pirh2 to either histone acetylase TIP60, PLAGL2 (Pleomorphic Adenoma Gene Like 2), or measles virus phosphoprotein (MV P protein) was reported to efficiently increase its stability by inhibiting polyubiquitination of Pirh2.3 Collectively, the activities of Pirh2 are controlled at multiple levels, enabling its quick and accurate adjustment in protein level depending on specific needs. It was reported that phosphorylated Pirh2 is far more unstable than its unphosphorylated form. Calmodulin-dependent kinase II (CaMK II) phosphorylates Pirh2 on residues Thr-154 and Ser-155. Phosphorylation of Pirh2 appears to be regulated through cell cycle-dependent mechanism. CaMK II-mediated Pirh2 phosphorylation abrogates its E3 ligase activity toward p53.4 Clinically it is revealed that increased expression of Pirh2 was correlated with poor survival in various cancer patients, indicating that Pirh2 is a novel prognostic marker for cancer.5
As a short-lived protein, Pirh2 is also a target for RING domain-dependent proteasomal degradation. Binding of Pirh2 to either histone acetylase TIP60, PLAGL2 (Pleomorphic Adenoma Gene Like 2), or measles virus phosphoprotein (MV P protein) was reported to efficiently increase its stability by inhibiting polyubiquitination of Pirh2.3 Collectively, the activities of Pirh2 are controlled at multiple levels, enabling its quick and accurate adjustment in protein level depending on specific needs. It was reported that phosphorylated Pirh2 is far more unstable than its unphosphorylated form. Calmodulin-dependent kinase II (CaMK II) phosphorylates Pirh2 on residues Thr-154 and Ser-155. Phosphorylation of Pirh2 appears to be regulated through cell cycle-dependent mechanism. CaMK II-mediated Pirh2 phosphorylation abrogates its E3 ligase activity toward p53.4 Clinically it is revealed that increased expression of Pirh2 was correlated with poor survival in various cancer patients, indicating that Pirh2 is a novel prognostic marker for cancer.5
REFERENCES
1. Yan, J. et al: FEBS lett. 17:24, 2010
2. Abe, K. et al: Biomed. Res. 29:53-61, 2008
3. Zheng, G. et al: Biochem. Biophy. Res. Commun. 354:344-50, 2007
4. Duan, S. et al: EMBO J. 26:3062-74, 2007
5. Wang, X-M. et al: Cancer 115:4554-63, 2009
2. Abe, K. et al: Biomed. Res. 29:53-61, 2008
3. Zheng, G. et al: Biochem. Biophy. Res. Commun. 354:344-50, 2007
4. Duan, S. et al: EMBO J. 26:3062-74, 2007
5. Wang, X-M. et al: Cancer 115:4554-63, 2009
Products are for research use only. They are not intended for human, animal, or diagnostic applications.
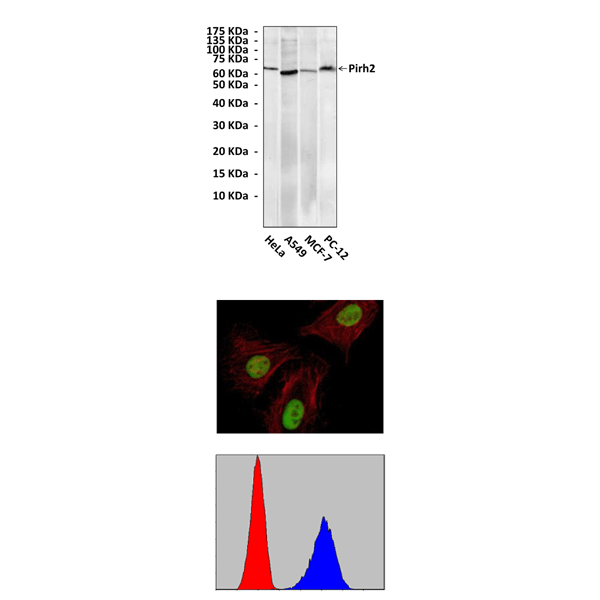
(Click to Enlarge) Top: Western Blot detection of Pirh2 proteins in various cell lysates using Pirh2 Antibody. Middle: This antibody stains HeLa cells in confocal immunofluorescent analysis. Bottom: It also specifically detects Pirh2 proteins in PC-12 cells in FACS testing (Pirh2 Antibody: Blue; control mouse IgG: Red).
Details
Cat.No.: | CP10201 |
Antigen: | Purified recombinant human Pirh2 fragments expressed in E. coli. |
Isotype: | Mouse IgG1 |
Species & predicted species cross- reactivity ( ): | Human, Mouse, Rat |
Applications & Suggested starting dilutions:* | WB 1:1000 IP n/d IHC n/d ICC 1:200 FACS 1:200 |
Predicted Molecular Weight of protein: | 32, 64 kDa |
Specificity/Sensitivity: | Detects endogenous Pirh2 proteins without cross-reactivity with other family members. |
Storage: | Store at -20°C, 4°C for frequent use. Avoid repeated freeze-thaw cycles. |
*Optimal working dilutions must be determined by end user.
Products
| Product | Size | CAT.# | Price | Quantity |
|---|---|---|---|---|
| Mouse p53-Inducible Protein with RING-H2 Domain Antibody: Mouse p53-Inducible Protein with RING-H2 Domain Antibody | Size: 100 ul | CAT.#: CP10201 | Price: $413.00 |
