Product Sheet CA9309
Description
BACKGROUND Phospholamban (PLN), a 52 amino acid phosphoprotein, is an effective inhibitor of the sarco(endo)plasmic reticulum Ca2+-ATPase, which transports Ca2+ into the sarcoplasmic reticulum (SR) lumen, leading to muscle relaxation. Sarco(endo)plasmic reticulum Ca2+-ATPases (SERCAs) are 110-kDa membrane proteins that transport Ca2+ from the cytosol actively to the lumen of the sarco(endo)plasmic reticulum. During cardiac action potential, Ca2+ enters the cell through voltage-dependent Ca2+ channels (L-type Ca2+ channels) and subsequently binds and activates the ryanodine receptors (RyR2) on SR, to trigger further Ca2+ release. This process, termed Ca2+-induced-Ca2+-release, amplifies and coordinates the Ca2+ signal, which, by interacting with myofilament proteins, produces contraction. To allow for muscle relaxation between contractions, cytosolic Ca2+ must be decreased rapidly. This is mainly accomplished by the SR Ca2+-ATPase (SERCA2a), which mediates Ca2+ uptake into the SR, and in less proportion by the Na+/Ca2+ exchanger (NCX), which removes Ca2+ from the extracellular space.1
The activity of SERCA2a is under the control of PLN. PLN, in the dephosphorylated form, decreases the apparent Ca2+-affinity of SERCA2a. The use of gene knockout and transgenic mouse models, in which the expression levels of PLN has been ablated, reduced or increased, constituted a crucial step in the recognition of the role of PLN in the regulation of myocardial contractility and relaxation. Ablation of PLN produced an enhanced contractility and relaxation. This hypercontractile function of PLN-deficient hearts was associated with increases in the apparent affinity of SERCA2a for Ca2+ and in the intraluminal SR Ca2+ content. In contrast, overexpression of PLN was associated with a decreased apparent affinity of SERCA2a for Ca2+ and depressed cardiac contractile performance. PLN-heterozygous hearts, expressing reduced protein levels of PLN, further support that the ratio PLN/SERCA2a plays a prominent role in regulating SR function and contractility. In addition to the relationship of PLN/SERCA2a, myocardial contractility and relaxation are also dependent on the degree of PLN phosphorylation. In vitro experiments have shown that PLN can be phosphorylated at three distinct sites: Ser16 by cAMP- and cGMP-dependent protein kinases (PKA and PKG, respectively), Thr17 by CaMKII, and Ser10 by PKC. Phosphorylation of these sites reverses the inhibition of PLN upon SERCA2a, thus increasing the affinity of the enzyme for Ca2+ and the rate of SR Ca2+ uptake. Experimental evidence indicated that phosphorylation of Ser10 by PKC is not physiologically relevant. Phosphorylation of Ser16 by PKG seems to play a role in the modulation of smooth muscle contraction and has been also associated with a positive inotropic and lusitropic effect in mammalian heart. Finally, phosphorylation of PLN by PKA and CaMKII pathways (Ser16 and Thr17 residues, respectively), is the main mediator of the positive inotropic and relaxant effect of beta-adrenergic stimulation in cardiac muscle. The increase in SERCA2a activity and Ca2+ uptake rate produced by these phosphorylations would lead to an increase in the velocity of relaxation, SR Ca2+ load and, as a consequence, SR Ca2+ release and myocardial contractility.2 The status of phosphorylation of PLN is also dependent on the activity of the type 1 phosphatase (PP1), the major SR-phosphatase that specifically dephosphorylates PLN. The activity of PP1 is also under the control of different kinases and phosphatases. This phosphatase regulatory cascade, frequently overlooked when considering the regulation of PLN phosphorylation sites, is crucial in determining the status of PLN phosphorylation.3 In addition, in fast twitch skeletal muscle SERCA1a associates with sarcolipin (SLN), a 31-amino acid protein which is an effective inhibitor of the SERCA molecule. PLN and SLN share significant amino acid sequence identity and gene structure and are clearly homologous members of a gene family.4
The activity of SERCA2a is under the control of PLN. PLN, in the dephosphorylated form, decreases the apparent Ca2+-affinity of SERCA2a. The use of gene knockout and transgenic mouse models, in which the expression levels of PLN has been ablated, reduced or increased, constituted a crucial step in the recognition of the role of PLN in the regulation of myocardial contractility and relaxation. Ablation of PLN produced an enhanced contractility and relaxation. This hypercontractile function of PLN-deficient hearts was associated with increases in the apparent affinity of SERCA2a for Ca2+ and in the intraluminal SR Ca2+ content. In contrast, overexpression of PLN was associated with a decreased apparent affinity of SERCA2a for Ca2+ and depressed cardiac contractile performance. PLN-heterozygous hearts, expressing reduced protein levels of PLN, further support that the ratio PLN/SERCA2a plays a prominent role in regulating SR function and contractility. In addition to the relationship of PLN/SERCA2a, myocardial contractility and relaxation are also dependent on the degree of PLN phosphorylation. In vitro experiments have shown that PLN can be phosphorylated at three distinct sites: Ser16 by cAMP- and cGMP-dependent protein kinases (PKA and PKG, respectively), Thr17 by CaMKII, and Ser10 by PKC. Phosphorylation of these sites reverses the inhibition of PLN upon SERCA2a, thus increasing the affinity of the enzyme for Ca2+ and the rate of SR Ca2+ uptake. Experimental evidence indicated that phosphorylation of Ser10 by PKC is not physiologically relevant. Phosphorylation of Ser16 by PKG seems to play a role in the modulation of smooth muscle contraction and has been also associated with a positive inotropic and lusitropic effect in mammalian heart. Finally, phosphorylation of PLN by PKA and CaMKII pathways (Ser16 and Thr17 residues, respectively), is the main mediator of the positive inotropic and relaxant effect of beta-adrenergic stimulation in cardiac muscle. The increase in SERCA2a activity and Ca2+ uptake rate produced by these phosphorylations would lead to an increase in the velocity of relaxation, SR Ca2+ load and, as a consequence, SR Ca2+ release and myocardial contractility.2 The status of phosphorylation of PLN is also dependent on the activity of the type 1 phosphatase (PP1), the major SR-phosphatase that specifically dephosphorylates PLN. The activity of PP1 is also under the control of different kinases and phosphatases. This phosphatase regulatory cascade, frequently overlooked when considering the regulation of PLN phosphorylation sites, is crucial in determining the status of PLN phosphorylation.3 In addition, in fast twitch skeletal muscle SERCA1a associates with sarcolipin (SLN), a 31-amino acid protein which is an effective inhibitor of the SERCA molecule. PLN and SLN share significant amino acid sequence identity and gene structure and are clearly homologous members of a gene family.4
REFERENCES
1. Mattiazzi, A. et al: Cardiovasc. Res. 68:366-75, 2005
2. Vittone, L. et al: Front. Biosci. 13:5988-6005, 2008
3. Mattiazzi, A. & Kranias, E.G.: Heart Rhythm. 2010 (in press)
4. Sharma, P. et al: PLoS ONE 5:e11496, 2010
2. Vittone, L. et al: Front. Biosci. 13:5988-6005, 2008
3. Mattiazzi, A. & Kranias, E.G.: Heart Rhythm. 2010 (in press)
4. Sharma, P. et al: PLoS ONE 5:e11496, 2010
Products are for research use only. They are not intended for human, animal, or diagnostic applications.
Details
Cat.No.: | CA9309 |
Antigen: | Short peptide from human PLN sequence. |
Isotype: | Rabbit IgG |
Species & predicted species cross- reactivity ( ): | Human, Mouse, Rat |
Applications & Suggested starting dilutions:* | WB 1:1000 IP n/d IHC 1:50 ICC n/d FACS n/d |
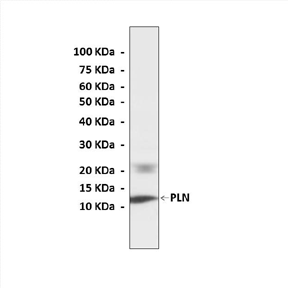
Predicted Molecular Weight of protein: | 10 kDa |
Specificity/Sensitivity: | detects endogenous levels of PLN proteins without cross-reactivity with other related proteins. |
Storage: | Store at -20°C, 4°C for frequent use. Avoid repeated freeze-thaw cycles. |
*Optimal working dilutions must be determined by end user.
Products
| Product | Size | CAT.# | Price | Quantity |
|---|---|---|---|---|
| Rabbit Phospholamban Antibody: Rabbit Phospholamban Antibody | Size: 100 ul | CAT.#: CA9309 | Price: $375.00 |