Product Sheet CP10266
Description
BACKGROUND The innate immune system is essential for host defense and is responsible for early detection of potentially pathogenic microorganisms. Upon recognition of microbes by innate immune cells such as macrophages and dendritic cells, diverse signaling pathways are activated that combine to define inflammatory responses that direct sterilization of the threat and/or orchestrate development of the adaptive immune response. Innate immune signaling must be carefully controlled, and regulation comes in part from interactions between activating and inhibiting signaling receptors. Toll-like receptors (TLR) have recently emerged as key receptors responsible for recognizing specific conserved components of microbes including lipopolysaccharides from Gram-negative bacteria, CpG DNA, and flagellin. Full activation of inflammatory responses by TLR may require the assembly of receptor signaling complexes including other transmembrane proteins that may influence signal transduction. In addition to TLR, many additional receptors participate in innate recognition of microbes, and recent studies demonstrate strong interactions between signaling through these receptors and signaling through TLR.1
Toll-like receptor 2 (TLR2) is a member of the vertebrate protein family of TLRs that has been studied in substantial detail over the last years. The extracellular domain of the type I receptor molecule TLR2 contains 18 to 20 leucine rich repeat (LRR) and LRR like motives. The intracellular domain of TLR2 contains a Toll/IL-1 receptor/resistance protein typical TIR domain. After the first implication of TLR4 in immunity thereinafter followed by the discovery of the lipopolysaccharide signal transducer function of TLR4, TLR2 was the first of ten mammalian TLRs proven to be directly involved in recognition of pathogen associated molecular patterns (PAMPs). Among the TLR2 specific agonists are microbial products representing broad groups of species such as Gram-positive and Gram-negative bacteria, as well as mycobacteria, spirochetes, and mycoplasm. PAMP induced phagosomal localization of TLR2 and TLR2 dependent apoptosis have been shown. Complex formation with other molecules involved in pattern recognition such as CD14, MD2, TLR1, and TLR6 has been implicated for TLR2. It was shown that gram-positive bacteria and their PGN component induce IL-8 production by activating the TLR2→MyD88→IRAK→TRAF6→NIK→IKK→NF-κB signal transduction pathway.2 In addition, TLR2 also participates in regulation of adaptive immune response. It was reported that TLR2 engagement on CD8 T cells decreases the activation threshold for co-stimulatory signals delivered by APC. TLR2 signaling induced splenic dendritic cells (DCs) to express the retinoic acid (RA) metabolizing enzyme Raldh2 and IL-10, and to metabolize vitamin A and stimulate Foxp3+ T regulatory cells
(Treg cells). RA acted on DCs to induce Socs3 expression, which suppressed activation of p38 MAPK and pro-inflammatory cytokines. Consistent with this, TLR2 signaling induced Treg cells, and suppressed IL-23 and TH-17/TH-1 mediated autoimmune responses in vivo.3 Surprisingly even proteinaceous host material such as heat shock protein (HSP) 60 has been demonstrated to activate cells through TLR2. Thus, TLR2 may be a sensor and inductor of specific defense processes, including oxidative stress and cellular necrosis initially spurred by microbial compounds. Detailed understanding of the biology of TLR2 will probably contribute to the characterization of a number of infectious diseases and potentially help in the development of novel intervention strategies.4
Toll-like receptor 2 (TLR2) is a member of the vertebrate protein family of TLRs that has been studied in substantial detail over the last years. The extracellular domain of the type I receptor molecule TLR2 contains 18 to 20 leucine rich repeat (LRR) and LRR like motives. The intracellular domain of TLR2 contains a Toll/IL-1 receptor/resistance protein typical TIR domain. After the first implication of TLR4 in immunity thereinafter followed by the discovery of the lipopolysaccharide signal transducer function of TLR4, TLR2 was the first of ten mammalian TLRs proven to be directly involved in recognition of pathogen associated molecular patterns (PAMPs). Among the TLR2 specific agonists are microbial products representing broad groups of species such as Gram-positive and Gram-negative bacteria, as well as mycobacteria, spirochetes, and mycoplasm. PAMP induced phagosomal localization of TLR2 and TLR2 dependent apoptosis have been shown. Complex formation with other molecules involved in pattern recognition such as CD14, MD2, TLR1, and TLR6 has been implicated for TLR2. It was shown that gram-positive bacteria and their PGN component induce IL-8 production by activating the TLR2→MyD88→IRAK→TRAF6→NIK→IKK→NF-κB signal transduction pathway.2 In addition, TLR2 also participates in regulation of adaptive immune response. It was reported that TLR2 engagement on CD8 T cells decreases the activation threshold for co-stimulatory signals delivered by APC. TLR2 signaling induced splenic dendritic cells (DCs) to express the retinoic acid (RA) metabolizing enzyme Raldh2 and IL-10, and to metabolize vitamin A and stimulate Foxp3+ T regulatory cells
(Treg cells). RA acted on DCs to induce Socs3 expression, which suppressed activation of p38 MAPK and pro-inflammatory cytokines. Consistent with this, TLR2 signaling induced Treg cells, and suppressed IL-23 and TH-17/TH-1 mediated autoimmune responses in vivo.3 Surprisingly even proteinaceous host material such as heat shock protein (HSP) 60 has been demonstrated to activate cells through TLR2. Thus, TLR2 may be a sensor and inductor of specific defense processes, including oxidative stress and cellular necrosis initially spurred by microbial compounds. Detailed understanding of the biology of TLR2 will probably contribute to the characterization of a number of infectious diseases and potentially help in the development of novel intervention strategies.4
REFERENCES
1. Underhill, D.M.: Eur. J. Immunol. 33:1767-75, 2003
2. Wang, Q. et al: Infect. Immun. 69:2270-6, 2001
3. Manicassamy, S. et al: Nat. Med. 15:401-9, 2009
4. Kirschning, C.J & Schumann, R.R.: Curr. Top. Microbiol. Immun. 270:1221-44, 2002
2. Wang, Q. et al: Infect. Immun. 69:2270-6, 2001
3. Manicassamy, S. et al: Nat. Med. 15:401-9, 2009
4. Kirschning, C.J & Schumann, R.R.: Curr. Top. Microbiol. Immun. 270:1221-44, 2002
Products are for research use only. They are not intended for human, animal, or diagnostic applications.
Details
Cat.No.: | CP10266 |
Antigen: | Purified recombinant human TLR2 fragments expressed in E. coli. |
Isotype: | Mouse IgG1 |
Species & predicted species cross- reactivity ( ): | Human, Mouse, Rat |
Applications & Suggested starting dilutions:* | WB 1:1000 IP n/d IHC n/d ICC n/d FACS n/d |
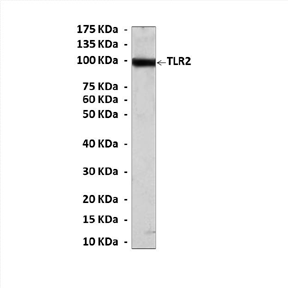
Predicted Molecular Weight of protein: | 100 kDa |
Specificity/Sensitivity: | Detects TLR2 proteins without cross-reactivity with other related proteins. |
Storage: | Store at -20°C, 4°C for frequent use. Avoid repeated freeze-thaw cycles. |
*Optimal working dilutions must be determined by end user.
Products
| Product | Size | CAT.# | Price | Quantity |
|---|---|---|---|---|
| Mouse Toll-Like Receptor 2 Antibody: Mouse Toll-Like Receptor 2 Antibody | Size: 100 ul | CAT.#: CP10266 | Price: $413.00 |