Product Sheet CA0577
Description
BACKGROUND The TIMPs are well-studied inhibitors of MMPs and consist of a family of four structurally related proteins (TIMP-1–4), with core proteins of -21 kDa. TIMPs inhibit MMP activity by a common mechanism involving interaction of the amino-terminal cysteine residue with the zinc atom at the MMP active site. The TIMPs inhibit MMP activity associated with tumor invasion and angiogenesis. In addition to their MMP-inhibitory activity, it is now widely appreciated that TIMPs have direct effects on cellular behaviors such as cell growth, apoptosis, migration, and differentiation.1
The TIMP-3 polypeptide sequence is 37% and 42% similar to the sequences of TIMP-1 and TIMP-2, respectively. The TIMP-3 protein has 188 amino acids. It has a conserved glycosylation site near the C-terminus. Characterisation of the human recombinant TIMP-3 reveals that it has both a 27 kDa glycosylated and a 24 kDa unglycosylated species. Unlike the other TIMPs, which are soluble, TIMP-3 is unique in being a component of extracellular matrix (ECM). TIMP-3 is localised to the ECM in both its glycosylated and unglycosylated forms. In addition, TIMP-3 is the only TIMP to inhibit members of the ADAM (a disintegrin and metalloprotease domain) family such as tumor necrosis factor-alpha-converting enzyme; this may account for its ability to induce apoptosis. It is the only TIMP to inhibit shedding of L-selectin and interleukin-6 receptors. Moreover, TIMP-3 is the only TIMP directly implicated in a disease process: Ser-Cys mutants of TIMP-3 accumulate in Bruch\'s membrane of the eye and cause Sorsby\'s fundus dystrophy. TIMP-3 also promotes the detachment of transformed cells from the ECM and is involved in the formation, branching, and expansion of epithelial tubes and in regulating trophoblast invasion of the uterus.2 Mutations in the human TIMP-3 gene cause a dominantly inherited, adult-onset blindness (Sorsby\'s fundus dystrophy or SFD).3 It was demonstrated that increased expression of TIMP-3 resulted in a statistically significant suppression of tumor growth. Deposition of TIMP-3 in the surrounding ECM by tumor cells may inhibit tumor growth by preventing local expansion of tumor, retarding the release of growth factors sequestered in ECM, or inhibiting angiogenesis. TIMP-3 over-expression had no effect on the growth of the two tumor cell lines in vitro. Because recombinant TIMP-3 inhibits endothelial cell migration and tube formation in response to angiogenic factors, thus, the effect of TIMP-3 on tumor growth seen in this study may be a consequence of its angiostatic action.4
The TIMP-3 polypeptide sequence is 37% and 42% similar to the sequences of TIMP-1 and TIMP-2, respectively. The TIMP-3 protein has 188 amino acids. It has a conserved glycosylation site near the C-terminus. Characterisation of the human recombinant TIMP-3 reveals that it has both a 27 kDa glycosylated and a 24 kDa unglycosylated species. Unlike the other TIMPs, which are soluble, TIMP-3 is unique in being a component of extracellular matrix (ECM). TIMP-3 is localised to the ECM in both its glycosylated and unglycosylated forms. In addition, TIMP-3 is the only TIMP to inhibit members of the ADAM (a disintegrin and metalloprotease domain) family such as tumor necrosis factor-alpha-converting enzyme; this may account for its ability to induce apoptosis. It is the only TIMP to inhibit shedding of L-selectin and interleukin-6 receptors. Moreover, TIMP-3 is the only TIMP directly implicated in a disease process: Ser-Cys mutants of TIMP-3 accumulate in Bruch\'s membrane of the eye and cause Sorsby\'s fundus dystrophy. TIMP-3 also promotes the detachment of transformed cells from the ECM and is involved in the formation, branching, and expansion of epithelial tubes and in regulating trophoblast invasion of the uterus.2 Mutations in the human TIMP-3 gene cause a dominantly inherited, adult-onset blindness (Sorsby\'s fundus dystrophy or SFD).3 It was demonstrated that increased expression of TIMP-3 resulted in a statistically significant suppression of tumor growth. Deposition of TIMP-3 in the surrounding ECM by tumor cells may inhibit tumor growth by preventing local expansion of tumor, retarding the release of growth factors sequestered in ECM, or inhibiting angiogenesis. TIMP-3 over-expression had no effect on the growth of the two tumor cell lines in vitro. Because recombinant TIMP-3 inhibits endothelial cell migration and tube formation in response to angiogenic factors, thus, the effect of TIMP-3 on tumor growth seen in this study may be a consequence of its angiostatic action.4
REFERENCES
1. Gomez, D.E.: Eur J Cell Biol. 74:111-22, 1997
2. Yu, W.H. et al: J. Biol. Chem. 275:31226-32, 2000
3. Weber, B.H.F. et al: Nature Genetics 8:352-56, 1994
4. Anand-Apte, B. et al: Biochem. Cell Biol. 74:853–62, 1996
2. Yu, W.H. et al: J. Biol. Chem. 275:31226-32, 2000
3. Weber, B.H.F. et al: Nature Genetics 8:352-56, 1994
4. Anand-Apte, B. et al: Biochem. Cell Biol. 74:853–62, 1996
Products are for research use only. They are not intended for human, animal, or diagnostic applications.
Details
Cat.No.: | CA0577 |
Antigen: | C- terminal sequence of human TIMP-3 |
Isotype: | Affinity-Purified Rabbit Polyclonal IgG |
Species & predicted species cross- reactivity ( ): | Human, Rat, Mouse |
Applications & Suggested starting dilutions:* | WB 1:500 - 1:1000 IP n/d IHC (Paraffin) 1:50 - 1:200 ICC n/d FACS n/d |
Predicted Molecular Weight of protein: | 24 kDa |
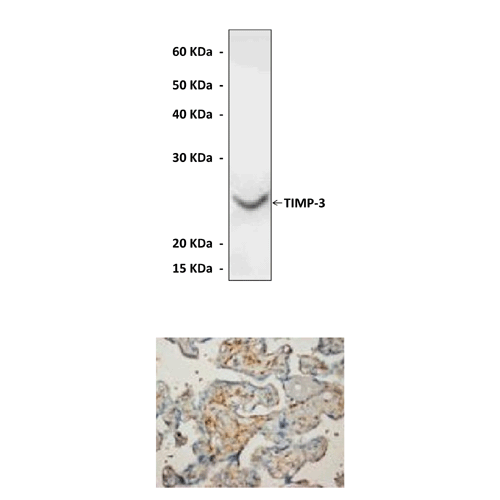
Specificity/Sensitivity: | Reacts specifically with TIMP-3 of human, mouse, and rat origin in immunohistochemical staining and western blotting, no cross-reactivity with other members of the family. |
Storage: | Store at 4° C for frequent use; at -20° C for at least one year. |
*Optimal working dilutions must be determined by end user.
Products
| Product | Size | CAT.# | Price | Quantity |
|---|---|---|---|---|
| Polyclonal Tissue Inhibitors of Metalloproteinases-3 Antibody: Polyclonal Tissue Inhibitors of Metalloproteinases-3 Antibody | Size: 100 ul | CAT.#: CA0577 | Price: $375.00 |