Product Sheet CP10362
Description
BACKGROUND Coactivator-associated arginine methyltransferase 1 (CARM1) belongs to the protein arginine methyltransferase (PRMT) family. As a type I PRMT enzyme, CARM1 catalyzes the transfer of methyl groups from S-adenosyl-l-methionine to the guanidine nitrogens of arginine, producing asymmetric dimethylated arginine as its final product. This is in contrast to the type II PRMT enzyme, which produces symmetric dimethylated arginine as its final product. CARM1 can function as a secondary coactivator for nuclear receptor-mediated transcription by methylating arginine residues 17 and 26 of histone H3. The indispensable role of CARM1 in the estrogen signaling pathway has been established by loss of estrogen receptor (ER) activity in CARM1-deficient mouse embryonic fibroblast cells. In addition to modifying the core histones at promoters, CARM1 can regulate the functions of transcriptional coactivators by methylation of their arginine residues. For instance, CARM1 can methylate various arginines in p300/CBP and thereby modulate the interactions between p300/CBP, steroid receptors, and cAMP-response element-binding protein. The combined results indicate that CARM1 is a dual functional coactivator that facilitates transcription initiation by methylation of histone H3 and also dictates the subsequent coactivator complex disassembly by methylation of SRC-3 and p300/CBP.1 Besides histones and transcriptional coregulators, CARM1 also can methylate RNA binding proteins such as PABP1, HuR, HuD, and thymocyte cyclic AMP-regulated phosphoprotein, as well as splicing factors such as CA150, SAP49, and SmB. The broad range of CARM1 substrates correlates with its functional diversity. It has been reported that CARM1 plays an important role in alternative splicing through methylation of the splicing factors. It also has been shown that CARM1 plays a critical role in promoting differentiation of early thymocyte progenitors, possibly through methylating thymocyte cyclic AMP-regulated phosphoprotein. Furthermore, it was reported that histone H3 arginine methylation by CARM1 in ES cells allows epigenetic modulation of pluripotency.2 Carm1 was also found to be involved in promoting adipocyte, epithelial and muscle cell differentiation. In addition, CARM1 has been shown to directly regulate expression of cell growth genes such as E2F1 and cyclin E1. Consistently, CARM1 has been implicated in tumorigenesis in several studies. For instance, overexpression of CARM1 was involved in the development of prostate carcinoma as well as androgen-independent prostate carcinoma, and the mRNA level of CARM1 was found to be elevated in grade 3 breast tumors. It has been proposed that CARM1 may represent a novel therapeutic target in cancers.
The CARM1 enzymatic functions are regulated by different physiological signaling pathways. It was shown that CARM1 was indeed phosphorylated at Ser217 both in vivo and in vitro and that this phosphorylation inactivated CARM1 methyltransferase activity by disrupting a hydrogen bond with Tyr154 and caused cytoplasmic localization of CARM1 protein. Interestingly, phosphorylation of CARM1 at Ser217 appears to occur mainly during cell mitosis, suggesting that phosphorylation at Ser217 serves as a molecular switch for controlling CARM1 enzymatic activity during the cell cycle.3 Moreover, it was also shown that the phosphorylation of the CARM1 by PKA at a single serine is necessary and sufficient for direct binding to the unliganded hormone-binding domain (HBD) of ERα, and the interaction is necessary for cAMP activation of ERα.4 In addition, it was shown that automethylation of CARM1 allows coupling of transcription and mRNA splicing.5
The CARM1 enzymatic functions are regulated by different physiological signaling pathways. It was shown that CARM1 was indeed phosphorylated at Ser217 both in vivo and in vitro and that this phosphorylation inactivated CARM1 methyltransferase activity by disrupting a hydrogen bond with Tyr154 and caused cytoplasmic localization of CARM1 protein. Interestingly, phosphorylation of CARM1 at Ser217 appears to occur mainly during cell mitosis, suggesting that phosphorylation at Ser217 serves as a molecular switch for controlling CARM1 enzymatic activity during the cell cycle.3 Moreover, it was also shown that the phosphorylation of the CARM1 by PKA at a single serine is necessary and sufficient for direct binding to the unliganded hormone-binding domain (HBD) of ERα, and the interaction is necessary for cAMP activation of ERα.4 In addition, it was shown that automethylation of CARM1 allows coupling of transcription and mRNA splicing.5
REFERENCES
1. Lee, Y.H. et al. Genes Dev. 25:176-88, 2011
2. Wu, Q. et al: Stem Cells 27:2637-45, 2009
3. Feng, Q. et al: J. Biol. Chem. 284:36167-74, 2009
4. Carascossa, S. et al: Genes Dev. 24:708-19, 2010
5. Kuhn, P. et al: Nucleic. Acids Res. 2010 (in press)
2. Wu, Q. et al: Stem Cells 27:2637-45, 2009
3. Feng, Q. et al: J. Biol. Chem. 284:36167-74, 2009
4. Carascossa, S. et al: Genes Dev. 24:708-19, 2010
5. Kuhn, P. et al: Nucleic. Acids Res. 2010 (in press)
Products are for research use only. They are not intended for human, animal, or diagnostic applications.
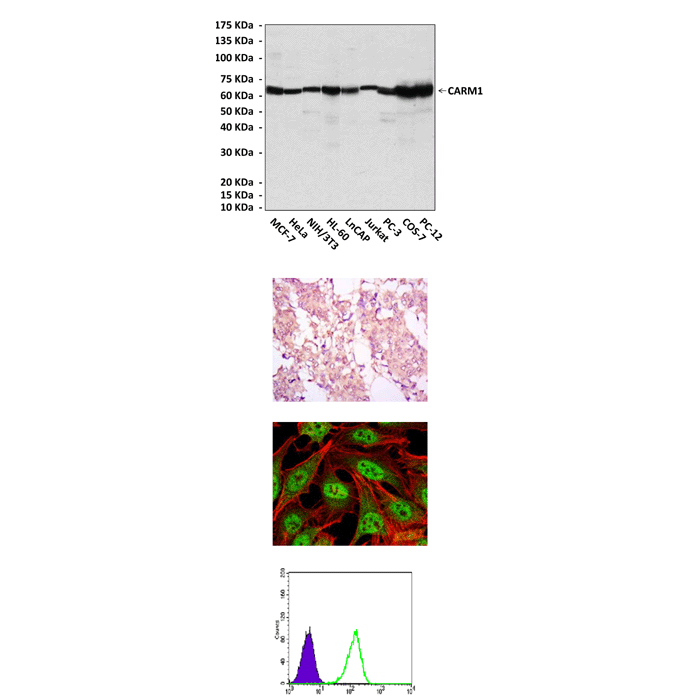
(Click to Enlarge) Top: Western blot detection of CARM1 proteins in various cell lysates using CARM1 antibody. Middle, upper: This antibody stains paraffin-embedded human ovarian cancer tissue in immunohistochemical analysis. Middle, lower: It also stains HeLa cells in confocal immunofluorescent assay (CARM1 Antibody: Green; Actin filaments: Red; DRAQ5 DNA Dye: Blue). Bottom: FACS analysis of Lovo cells using CARM1 Antibody (green) and negative control (purple).
Details
Cat.No.: | CP10362 |
Antigen: | Raised against recombinant human CARM1 fragments expressed in E. coli. |
Isotype: | Mouse IgG1 |
Species & predicted species cross- reactivity ( ): | Human, Mouse, Rat |
Applications & Suggested starting dilutions:* | WB 1:1000 IP n/d IHC 1:50 - 1:200 ICC 1:50 - 1:200 FACS n/d |
Predicted Molecular Weight of protein: | 65 kDa |
Specificity/Sensitivity: | Detects CARM1 proteins in various cell lysate. |
Storage: | Store at -20°C, 4°C for frequent use. Avoid repeated freeze-thaw cycles. |
*Optimal working dilutions must be determined by end user.
Products
| Product | Size | CAT.# | Price | Quantity |
|---|---|---|---|---|
| Mouse Coactivator-Associated Arginine Methyltransferase 1 Antibody: Mouse Coactivator-Associated Arginine Methyltransferase 1 Antibody | Size: 100 ul | CAT.#: CP10362 | Price: $457.00 |
