Product Sheet CP10375
Description
BACKGROUND Ribosomal protein synthesis is one of the oldest and best-conserved processes taking place in a living cell. Amino acid residues are added to the growing peptide chain in the ribosome by an elongation process that involves two GTP-switched elongation factors, denominated EF1 and EF2 in eukaryotes. EF1-GTP brings the aminoacyl-tRNA (as the so-called ternary complex) to the acceptor site on the ribosome. After the nascent protein chain is transpeptidated to the newly arrived tRNA, EF2 catalyzes a conformational switch of the organelle, such that the newly generated peptidyl-tRNA is moved from the acceptor site to the peptidyl site, liberating the former for a new round of elongation. EF2 is a large (more than 800-residue), probably multifunctional, and remarkable protein that apparently binds to the same ribosomal structures as the EF1-GTP–aminoacyl-tRNA complex.
eEF2 (eukaryotic translation elongation factor 2) is a member of the GTP-binding translation elongation factor family. This protein is an essential factor for protein synthesis. It promotes the GTP-dependent translocation of the nascent protein chain from the A-site to the P-site of the ribosome. eEF2 is phosphorylated and inhibited by a calcium/calmodulin-dependent protein kinase called eEF2 kinase, which modifies Thr56 and Thr58. Phosphorylation at Thr56 and Thr58 results in the inactivation of eEF2 by causing a structural alteration that reduces its affinity for the ribosome, thereby preventing its ability to catalyze translocation.1 eEF2 kinase is also subjected to regulation by phosphorylation, and a number of phosphorylation sites have been identified that lead to subsequent activation or inhibition of activity.2,3
eEF2 (eukaryotic translation elongation factor 2) is a member of the GTP-binding translation elongation factor family. This protein is an essential factor for protein synthesis. It promotes the GTP-dependent translocation of the nascent protein chain from the A-site to the P-site of the ribosome. eEF2 is phosphorylated and inhibited by a calcium/calmodulin-dependent protein kinase called eEF2 kinase, which modifies Thr56 and Thr58. Phosphorylation at Thr56 and Thr58 results in the inactivation of eEF2 by causing a structural alteration that reduces its affinity for the ribosome, thereby preventing its ability to catalyze translocation.1 eEF2 kinase is also subjected to regulation by phosphorylation, and a number of phosphorylation sites have been identified that lead to subsequent activation or inhibition of activity.2,3
REFERENCES
1. Redpath, N.T. et al: Eur. J. Biochem. 213:689-99, 1993
2. Knebel, A. et al: EMBO J. 20:4360-9, 2001
3. Rose, A.J. et al: J. Physiol. 587:1547-63, 2009
2. Knebel, A. et al: EMBO J. 20:4360-9, 2001
3. Rose, A.J. et al: J. Physiol. 587:1547-63, 2009
Products are for research use only. They are not intended for human, animal, or diagnostic applications.
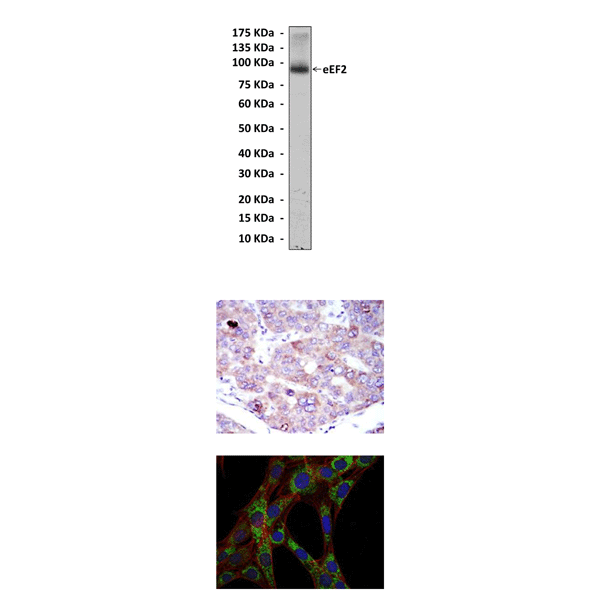
(Click to Enlarge) Top: Western Blot detection of eEF2 proteins in HepG2 cell lysate using eEF2 Antibody. Middle: This antibody stains paraffin-embedded human hepatoma tissue in immunohistochemical analysis. Bottom: It also stains 3T3-L1 cells in confocal immunofluorescent studies (eEF2 Antibody: Green; Actin filaments: Red; DRAQ5 DNA Dye: Blue).
Details
Cat.No.: | CP10375 |
Antigen: | Raised against purified recombinant fragments of human eEF2 expressed in E. Coli. |
Isotype: | Mouse IgG1 |
Species & predicted species cross- reactivity ( ): | Human |
Applications & Suggested starting dilutions:* | WB 1:1000 IP n/d IHC 1:50 - 1:200 ICC 1:50 - 1:200 FACS n/d |
Predicted Molecular Weight of protein: | 95 kDa |
Specificity/Sensitivity: | Detects eEF2 proteins without cross-reactivity with other family members. |
Storage: | Store at -20°C, 4°C for frequent use. Avoid repeated freeze-thaw cycles. |
*Optimal working dilutions must be determined by end user.
Products
| Product | Size | CAT.# | Price | Quantity |
|---|---|---|---|---|
| Mouse eEF2 Antibody: Mouse eEF2 Antibody | Size: 100 ul | CAT.#: CP10375 | Price: $457.00 |
