Product Sheet CP10380
Description
BACKGROUND Human HSP60, the product of the HSPD1 gene, is a Group I mitochondrial chaperonin, phylogenetically related to bacterial GroEL. HSP60 has long been known as an important chaperonin and as having key folding functions within the mitochondria. It is involved in mitochondrial protein import and macromolecular assembly and facilitates the correct folding of imported proteins. It may also prevent misfolding and promote the refolding and proper assembly of unfolded polypeptides generated in the mitochondrial matrix under stress conditions.1
Recently, the presence of HSP60 outside the mitochondria and outside the cell, e.g. in circulating blood, has been reported.2 This extra-mitochondrial HSP60 in the heart has key anti-apoptotic functions. Extra-mitochondrial HSP60 complexes with both bax and bak, but not with bcl-2. Reduction in HSP60 is sufficient to precipitate apoptosis. However, HSP60 may participate in triggering apoptosis in other cells. In addition, HSP60 has been found on the cell-membrane\'s outer surface in both normal and tumor cells. It has been implicated in transmembrane transport and signaling, activation and maturation of dendritic cells with generation of antitumor T-cell responses. Microbial HSP60 constitutes a major antigen recognized by the immune system during bacterial infections. Owing to the strong homology between microbial and human HSP60 an immune reaction against infectious microbes may lead to a cross-reactive autoimmune response against the native human HSP60.3
Recently, the presence of HSP60 outside the mitochondria and outside the cell, e.g. in circulating blood, has been reported.2 This extra-mitochondrial HSP60 in the heart has key anti-apoptotic functions. Extra-mitochondrial HSP60 complexes with both bax and bak, but not with bcl-2. Reduction in HSP60 is sufficient to precipitate apoptosis. However, HSP60 may participate in triggering apoptosis in other cells. In addition, HSP60 has been found on the cell-membrane\'s outer surface in both normal and tumor cells. It has been implicated in transmembrane transport and signaling, activation and maturation of dendritic cells with generation of antitumor T-cell responses. Microbial HSP60 constitutes a major antigen recognized by the immune system during bacterial infections. Owing to the strong homology between microbial and human HSP60 an immune reaction against infectious microbes may lead to a cross-reactive autoimmune response against the native human HSP60.3
REFERENCES
1. Ostermann, J. et al: Nature 341:125-130, 1989
2. Merendino, A.N. et al: PLoS ONE 5:e9247, 2010
3. Birk, A.S. et al: Proc. Natl. Acad. Sci. USA 93:1032-7, 1996
2. Merendino, A.N. et al: PLoS ONE 5:e9247, 2010
3. Birk, A.S. et al: Proc. Natl. Acad. Sci. USA 93:1032-7, 1996
Products are for research use only. They are not intended for human, animal, or diagnostic applications.
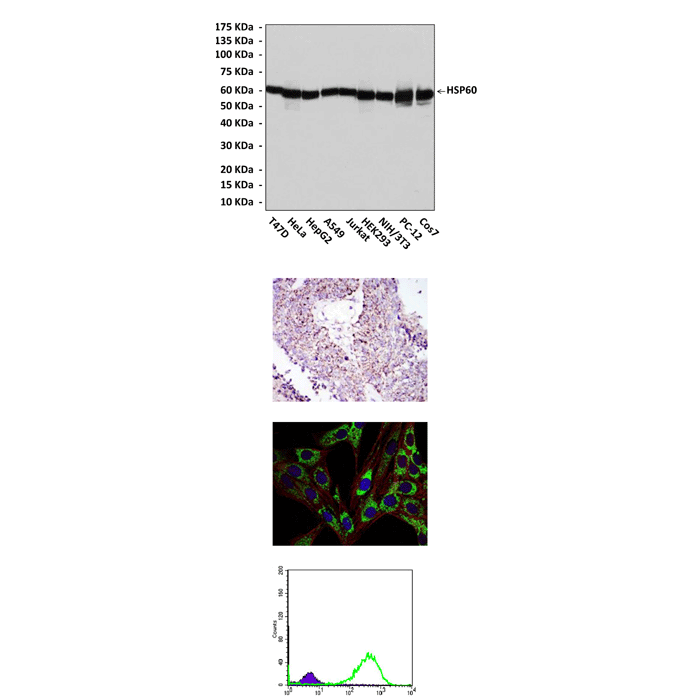
(Click to Enlarge) Top: Western Blot detection of HSP60 proteins in various cell lysates using HSP60 Antibody. Middle up: This antibody stains paraffin-embedded human lung cancer tissue in immunohistochemical analysis. Middle low: It also stains 3T3-L1 cells in confocal immunofluorescent studies (HSP60 Antibody: Green; Actin filaments: Red; DRAQ5 DNA Dye: Blue). Bottom: This antibody specifically reacts with HSP60 proteins in HeLa cells in FACS assay (HSP60 antibody: Green; Control: Purple).
Details
Cat.No.: | CP10380 |
Antigen: | Raised against purified recombinant fragments of human HSP60 expressed in E. Coli |
Isotype: | Mouse IgG1 |
Species & predicted species cross- reactivity ( ): | Human, Mouse, Rat |
Applications & Suggested starting dilutions:* | WB 1:1000 IP n/d IHC 1:50 - 1:200 ICC 1:50 - 1:200 FACS 1:50 - 1:200 |
Predicted Molecular Weight of protein: | 60 kDa |
Specificity/Sensitivity: | Detects HSP60 proteins without cross-reactivity with other family members. |
Storage: | Store at -20°C, 4°C for frequent use. Avoid repeated freeze-thaw cycles. |
*Optimal working dilutions must be determined by end user.
Products
| Product | Size | CAT.# | Price | Quantity |
|---|---|---|---|---|
| Mouse HSP60 Antibody: Mouse HSP60 Antibody | Size: 100 ul | CAT.#: CP10380 | Price: $457.00 |
