Product Sheet CG1539
Description
BACKGROUND Serum- and Glucocorticoid-Induced Kinase (SGK or SGK1) belong to a new family of serine/threonine kinases that are regulated at both the transcriptional and posttranslational levels by external stimuli. SGK family has also two other members SGK2 and SGK3. While SGK1 and SGK3 are expressed in all tissues tested so far, SGK2 is found only in some tissues including kidney, liver, pancreas and choroid plexus of the brain. The mRNA encoding SGK is rapidly induced in response to a variety of stimuli, including growth factors, steroid and peptide hormones, cytokines, changes in cell volume, and brain injury. SGK1 is known to regulate a variety of cellular functions, including salt homeostasis, ion channel conductance, cell proliferation, and neuronal excitability. In addition, SGK1 promotes cell survival and regulates cell cycle progression through phosphorylation of the forkhead transcription factor FKHRL1. More recently, SGK1 was found to modulate the excitatory amino acid transporter function through phosphorylation of SLC6A19.1 Moreover, SGK1 plays an important role in activating certain potassium, sodium, and chloride channels by phosphorylation of Nedd4-2. It was found that SGK1 regulates neuronal plasticity by phosphorylating IKKalpha and p300, and up-regulating the expression of the N-methyl-d-aspartate (NMDA) receptor subunit NR2A and NR2B.2
Within the protein kinase superfamily, SGK is closely related to Akt. Like Akt, The activation of SGK is dependent upon PI-3 Kinase activity and requires the phosphorylation of two regulatory sites, Thr256 and Ser422 (the equivalent phosphorylation sites for SGK2 and SGK3 are the residues Thr193/ Ser 356 andThr253/ Ser 419, respectively), that lie in the activation loop and the C-terminal domain of SGK, respectively. The protein kinase PDK1 is responsible for the phosphorylation of SGK at Thr256. The kinase that phosphorylates Ser422 has be identified as mTORC2.3 SGK is also a downstream target of the MAPK/ERK signaling cascade. ERK directly phosphorylates SGK at Ser78 and indirectly activates SGK at Thr256 and Ser422 through unknown intermediate molecules.4 Upon activation by growth factors, endogenous SGK translocates rapidly into the nucleus, where it may encounter nuclear substrates. SGK and Akt are likely to phosphorylate related substrates, as SGK has a consensus motif (KKRNRRLSVA) similar to that of Akt (RXRXXS/T). It has been demonstrated that both Akt and SGK can cooperate to phosphorylate substrates including GSK3-beta, B-Raf , p27 and FKHRL1 etc. Despite their similarity, SGK and Akt display unique features. These two kinases might have complementary rather than redundant functions. In addition, SGK is modified by polyubiquitination and ultimately degraded by the 26S proteasome. The rapid degradation of SGK suggests that in addition to transcriptional up-regulation and reversible phosphorylation, ubiquitin modification plays an important role in determining the availability of SGK as a kinase.5
Within the protein kinase superfamily, SGK is closely related to Akt. Like Akt, The activation of SGK is dependent upon PI-3 Kinase activity and requires the phosphorylation of two regulatory sites, Thr256 and Ser422 (the equivalent phosphorylation sites for SGK2 and SGK3 are the residues Thr193/ Ser 356 andThr253/ Ser 419, respectively), that lie in the activation loop and the C-terminal domain of SGK, respectively. The protein kinase PDK1 is responsible for the phosphorylation of SGK at Thr256. The kinase that phosphorylates Ser422 has be identified as mTORC2.3 SGK is also a downstream target of the MAPK/ERK signaling cascade. ERK directly phosphorylates SGK at Ser78 and indirectly activates SGK at Thr256 and Ser422 through unknown intermediate molecules.4 Upon activation by growth factors, endogenous SGK translocates rapidly into the nucleus, where it may encounter nuclear substrates. SGK and Akt are likely to phosphorylate related substrates, as SGK has a consensus motif (KKRNRRLSVA) similar to that of Akt (RXRXXS/T). It has been demonstrated that both Akt and SGK can cooperate to phosphorylate substrates including GSK3-beta, B-Raf , p27 and FKHRL1 etc. Despite their similarity, SGK and Akt display unique features. These two kinases might have complementary rather than redundant functions. In addition, SGK is modified by polyubiquitination and ultimately degraded by the 26S proteasome. The rapid degradation of SGK suggests that in addition to transcriptional up-regulation and reversible phosphorylation, ubiquitin modification plays an important role in determining the availability of SGK as a kinase.5
REFERENCES
1. Bohmer, C. et al: Cell. Physiol. Biochem. 25:723-32, 2010
2. Tai, D.J.C. et al: J. Biol. Chem. 284:4073-89, 2009
3. Yan, L. et al: Biochem. J. 416:e19-e21, 2008
4. Lee, C.T. et al: Eur. J. Neurosci. 23:1311-20, 2006
5. Brickley, D.R. et al: J. Biol. Chem. 277:43064-70, 2002
2. Tai, D.J.C. et al: J. Biol. Chem. 284:4073-89, 2009
3. Yan, L. et al: Biochem. J. 416:e19-e21, 2008
4. Lee, C.T. et al: Eur. J. Neurosci. 23:1311-20, 2006
5. Brickley, D.R. et al: J. Biol. Chem. 277:43064-70, 2002
Products are for research use only. They are not intended for human, animal, or diagnostic applications.
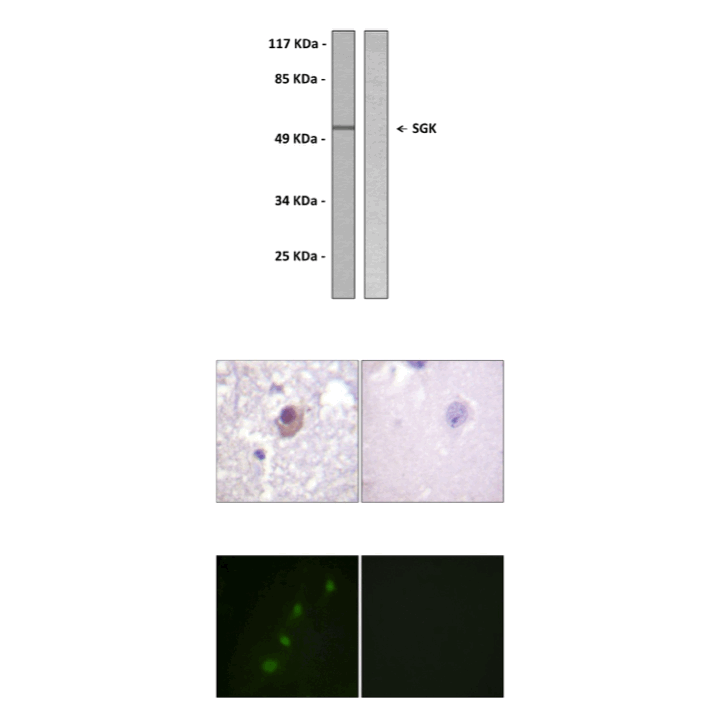
(Click to Enlarge) Top: Immunoblotting analysis of extracts from 293 cells, treated with heat shock, using Anti-SGK antibody. The lane on the left was treated with the Anti-SGK antibody. The lane on the right (negative control) was treated with both Anti-SGK antibody and the synthesized immunogen peptide. Middle: Immunohistochemistry analysis of paraffin-embedded human brain tissue using Anti-SGK antibody. Cells on the left were treated with the Anti-SGK antibody. Cells on the right (negative control) were treated with both Anti-SGK antibody and the synthesized immunogen peptide. Bottom: Immunofluorescence of HeLa cells using Anti-SGK antibody. Cells on the left were treated with the Anti-SGK antibody. Cells on the right (negative control) were treated with both Anti-SGK antibody and the synthesized immunogen peptide.
Details
Cat.No.: | CG1539 |
Antigen: | Synthesized peptide derived from human SGK |
Isotype: | Rabbit IgG |
Species & predicted species cross- reactivity ( ): | Human, Rat, Mouse |
Applications & Suggested starting dilutions:* | WB 1:500-1:1000 IP n/d IHC 1:50-1:1000 ICC n/d FACS n/d ELISA 1:100-1:500 |
Predicted Molecular Weight of protein: | 48 kDa |
Specificity/Sensitivity: | Detects endogenous SGK proteins without cross-reactivity with other family members. |
Storage: | Store at -20°C, 4°C for frequent use. Avoid repeated freeze-thaw cycles. |
*Optimal working dilutions must be determined by end user.
Products
| Product | Size | CAT.# | Price | Quantity |
|---|---|---|---|---|
| Rabbit Serum and Glucocorticoid Induced Kinase Antibody: Rabbit Serum and Glucocorticoid Induced Kinase Antibody | Size: 100 ul | CAT.#: CG1539 | Price: $384.00 |
