Product Sheet CP10377
Description
BACKGROUND Histone H3 lysine 9 dimethylation (H3K9me2) is an important posttranslational modification associated with transcriptional silencing. In contrast to H3K9me3 that is localized in pericentric heterochromatin, H3K9me2 is mainly localized within the silent regions of the euchromatin. At least three methyltransferases, G9a, GLP-1 (EuHMTare), and ESET (SetDB1, or KMT1E), have been identified to be able to catalyze the formation of H3K9me2. G9a is the major histone methyltransferase for H3K9me2 formation, because knockout of this gene in mice causes a 90% loss of H3K9me2. Knockout of this gene resulted in embryonic lethality and the embryos failed to develop past E8.5. Although GLP-1 (EuHMTare) itself is also a methyltransferase for the formation of H3K9me2, it forms a heteromeric complex with G9a and its knockout phenocopied G9a deletion. Histone H3 lysine 27 dimethylation (H3K27me2) is also a repressive mark associated with transcriptional silencing. The Polycomb repressive complex 2 (PRC2) that contains Ezh2, Eed, and Suz12 catalyzes the formation of this mark. The three components of the PRC2 complex are all essential for embryonic development, since knockout of any one of them leads to severe defects during gastrulation. It was shown recently that that KIAA1718, a member of the PHF2/PHF8 family, is a histone demethylase specific for both H3K9me2 and H3K27me2.1
ERG-associated protein with SET domain (ESET), also known as SET domain bifurcated 1 (SETDB1) is a histone methyltransferase that catalyses a repressive mark on euchromatin by mediating the methylation of mono- and dimethylated states of histone H3 Lys 9 residue to form H3K9me2 and H3K9me3, respectively. These marks are generally associated with transcriptional silencing and are bound by corepressors such as HP1. The ESET protein contains a Tudor domain, a methyl-CpG binding domain and a bifurcated SET domain that is responsible for its catalytic activity. ESET is critical for very early development since the Eset-null embryos die at the peri-implantation stage with defective development of the inner cell mass (ICM), from which no embryonic stem (ES) cells could be derived. Thus, it is reasoned that Eset may play an important role in ES cell biology.2 The Eset-null phenotype is similar to that of Oct4-null embryos. It was shown that ESET maintains pluripotency through repression of Cdx2, a key trophectoderm determinant, by histone H3 lysine 9 trimethylation (H3K9me3) of the promoter region. Notably, this repression is mediated through the synergistic function of small ubiquitin-related modifier (SUMO)ylated ESET and Oct4. ESET localises to the promyelocytic leukaemia (PML) nuclear bodies and is SUMOylated in ES cells. Interaction of ESET with Oct4 depends on a SUMO-interacting motif (SIM) in Oct4, which is critical for the repression of Cdx2. Thus, SUMOylated ESET-Oct4 complex is critical for both the initiation and maintenance of pluripotency through repression of differentiation, particularly of the trophectoderm lineage by epigenetic silencing of Cdx2.3 Moreover, it was shown that a DNA-methylation-independent pathway involving KAP1 and ESET/ESET-mediated H3K9me3 is required for proviral silencing during the period early in embryogenesis when DNA methylation is dynamically reprogrammed.4 In addition, studies point to a role for neuronal ESET in the regulation of affective and motivational behaviors through repressive chromatin remodeling at a select set of target genes, resulting in altered NMDA receptor subunit composition and other molecular adaptations.5
ERG-associated protein with SET domain (ESET), also known as SET domain bifurcated 1 (SETDB1) is a histone methyltransferase that catalyses a repressive mark on euchromatin by mediating the methylation of mono- and dimethylated states of histone H3 Lys 9 residue to form H3K9me2 and H3K9me3, respectively. These marks are generally associated with transcriptional silencing and are bound by corepressors such as HP1. The ESET protein contains a Tudor domain, a methyl-CpG binding domain and a bifurcated SET domain that is responsible for its catalytic activity. ESET is critical for very early development since the Eset-null embryos die at the peri-implantation stage with defective development of the inner cell mass (ICM), from which no embryonic stem (ES) cells could be derived. Thus, it is reasoned that Eset may play an important role in ES cell biology.2 The Eset-null phenotype is similar to that of Oct4-null embryos. It was shown that ESET maintains pluripotency through repression of Cdx2, a key trophectoderm determinant, by histone H3 lysine 9 trimethylation (H3K9me3) of the promoter region. Notably, this repression is mediated through the synergistic function of small ubiquitin-related modifier (SUMO)ylated ESET and Oct4. ESET localises to the promyelocytic leukaemia (PML) nuclear bodies and is SUMOylated in ES cells. Interaction of ESET with Oct4 depends on a SUMO-interacting motif (SIM) in Oct4, which is critical for the repression of Cdx2. Thus, SUMOylated ESET-Oct4 complex is critical for both the initiation and maintenance of pluripotency through repression of differentiation, particularly of the trophectoderm lineage by epigenetic silencing of Cdx2.3 Moreover, it was shown that a DNA-methylation-independent pathway involving KAP1 and ESET/ESET-mediated H3K9me3 is required for proviral silencing during the period early in embryogenesis when DNA methylation is dynamically reprogrammed.4 In addition, studies point to a role for neuronal ESET in the regulation of affective and motivational behaviors through repressive chromatin remodeling at a select set of target genes, resulting in altered NMDA receptor subunit composition and other molecular adaptations.5
REFERENCES
1. Huang, C. et al: Cell Res. 20:154-65, 2010
2. Yuan, P. et al: Genes Dev. 23:2507-20, 2009
3. Yeap, L-S. et al: Epigenetics & Chromatin 2:12, 2009
4. Matsui, T. et al: Nature 646:927-31, 2010
5. Jiang, Y. et al: J. Neurosci. 30:7152-67, 2010
2. Yuan, P. et al: Genes Dev. 23:2507-20, 2009
3. Yeap, L-S. et al: Epigenetics & Chromatin 2:12, 2009
4. Matsui, T. et al: Nature 646:927-31, 2010
5. Jiang, Y. et al: J. Neurosci. 30:7152-67, 2010
Products are for research use only. They are not intended for human, animal, or diagnostic applications.
Details
Cat.No.: | CP10377 |
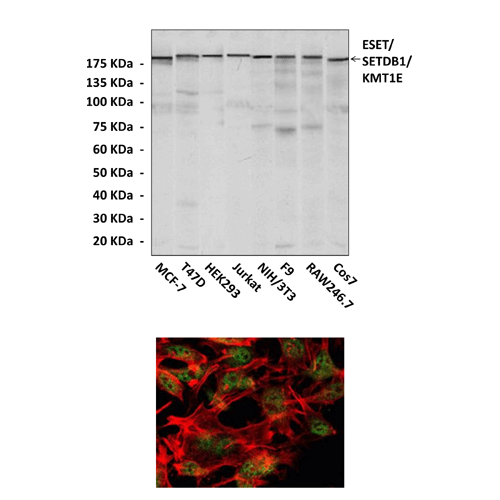
Antigen: | Raised against purified recombinant fragments of human ESET expressed in E. Coli. |
Isotype: | Mouse IgG1 |
Species & predicted species cross- reactivity ( ): | Human, Mouse, Rat |
Applications & Suggested starting dilutions:* | WB 1:1000 IP n/d IHC n/d ICC 1:50 - 1:200 FACS n/d |
Predicted Molecular Weight of protein: | 180 kDa |
Specificity/Sensitivity: | Detects ESET proteins without cross-reactivity with other family members. |
Storage: | Store at -20°C, 4°C for frequent use. Avoid repeated freeze-thaw cycles. |
*Optimal working dilutions must be determined by end user.
Products
| Product | Size | CAT.# | Price | Quantity |
|---|---|---|---|---|
| Mouse ESET/SETDB1/KMT1E Antibody: Mouse ESET/SETDB1/KMT1E Antibody | Size: 100 ul | CAT.#: CP10377 | Price: $413.00 |