Product Sheet CP10185
Description
BACKGROUND All known members of the Nerve Growth Factor (NGF) family, designated the neurotrophins, bind to two different types of receptors, the trk tyrosine kinases and the neurotrophin receptor p75NTR. p75NTR is a member of the Tumor Necrosis Factor (TNF) receptor and FAS/Apo-1/CD95 family. p75NTR contains tandem arrays of cysteine-rich domains (CRDs) in its extracellular portion. The CRDs in p75NTR are required for neurotrophin binding. TNFR superfamily members typically bind homotrimeric ligands that are produced as type II transmembrane proteins, and most act as independent signaling units. In contrast, p75NTR binds soluble dimeric ligands and often requires (or acts as) a coreceptor to activate biological activity. The death domain in p75NTR is structurally distinct from that in other TNFR superfamily members too, thus signaling properties of p75NTR are distinct from its TNFR brethrens.
It has revealed that p75NTR is a component of three distinct receptor platforms that bind different ligands and that, under differing circumstances, facilitate cell survival, cell death, or growth inhibition. At first, p75NTR physically interacts with the TrkA receptor and enhances the ability of TrkA to respond to NGF and discriminate between preferred and nonpreferred neurotrophin ligands.1 In the cells lacking of Trk receptors, p75NTR has been shown to mediate cell death in a ligand-dependent fashion. Upon activation, p75NTR assembles a signaling complex that may include NRAGE, NRIF, and other adaptors. Rac1 is activated and leads to activation of a JNK cascade that result in phosphorylation of Bad and perhaps other BH3 domain-only family members that release inhibition of Bax and Bak. Subsequent release of mitochondrial components that include SMAC and cytochrome C facilitates caspase activation. Moreover, it was discovered that p75NTR and Sortilin ormed a signaling complex mediating proapoptotic signals in response to proNGF binding. Finally, p75NTR may form a tripartite complex with the NogoR and with Lingo-1 that results in growth inhibitory signals to be transduced in response to Nogo, MAG, or OMgP.2 p75NTR mediates RhoA activation via a direct interaction with Rho-GDIα through this complex. When the complex is in an unliganded state, Rho-GDIα is associated with inactive Rho-GDP in the cytosol. Binding of MBGIs to the complex produces a conformational shift in the complex that allows Rho-GDIalpha to bind to the fifth helix of the p75NTR death domain and thereby release RhoA. Once released from Rho-GDIα, RhoA is able to exchange GDP for GTP and achieve its active conformation and activate downstream substrates. In addition, a role for p75NTR in neurite outgrowth has been shown in dorsal root ganglia and sympathetic neurons. In schwann cells and sensory neurons, NGF can activate nuclear factor (NF)-kappaB by binding to p75NTR. NF-kappaB is a part of a crucial survival pathway in NGF-dependent sympathetic neurons. In the presence of NGF, p75NTR can also rescue cells from apoptosis by activating NF-kappaB.3 In addition it was shown that ligand-dependent p75NTR regulation of the ceramide pathway mediates survival in certain neurons.4 It is worthwhile to notice that p75NTR is re-expressed in various pathological conditions, including epilepsy, axotomy and neurodegeneration. Potentially toxic peptides, including the amyloid beta-peptide that accumulates in Alzheimer\'s disease, are ligands for p75NTR.
It has revealed that p75NTR is a component of three distinct receptor platforms that bind different ligands and that, under differing circumstances, facilitate cell survival, cell death, or growth inhibition. At first, p75NTR physically interacts with the TrkA receptor and enhances the ability of TrkA to respond to NGF and discriminate between preferred and nonpreferred neurotrophin ligands.1 In the cells lacking of Trk receptors, p75NTR has been shown to mediate cell death in a ligand-dependent fashion. Upon activation, p75NTR assembles a signaling complex that may include NRAGE, NRIF, and other adaptors. Rac1 is activated and leads to activation of a JNK cascade that result in phosphorylation of Bad and perhaps other BH3 domain-only family members that release inhibition of Bax and Bak. Subsequent release of mitochondrial components that include SMAC and cytochrome C facilitates caspase activation. Moreover, it was discovered that p75NTR and Sortilin ormed a signaling complex mediating proapoptotic signals in response to proNGF binding. Finally, p75NTR may form a tripartite complex with the NogoR and with Lingo-1 that results in growth inhibitory signals to be transduced in response to Nogo, MAG, or OMgP.2 p75NTR mediates RhoA activation via a direct interaction with Rho-GDIα through this complex. When the complex is in an unliganded state, Rho-GDIα is associated with inactive Rho-GDP in the cytosol. Binding of MBGIs to the complex produces a conformational shift in the complex that allows Rho-GDIalpha to bind to the fifth helix of the p75NTR death domain and thereby release RhoA. Once released from Rho-GDIα, RhoA is able to exchange GDP for GTP and achieve its active conformation and activate downstream substrates. In addition, a role for p75NTR in neurite outgrowth has been shown in dorsal root ganglia and sympathetic neurons. In schwann cells and sensory neurons, NGF can activate nuclear factor (NF)-kappaB by binding to p75NTR. NF-kappaB is a part of a crucial survival pathway in NGF-dependent sympathetic neurons. In the presence of NGF, p75NTR can also rescue cells from apoptosis by activating NF-kappaB.3 In addition it was shown that ligand-dependent p75NTR regulation of the ceramide pathway mediates survival in certain neurons.4 It is worthwhile to notice that p75NTR is re-expressed in various pathological conditions, including epilepsy, axotomy and neurodegeneration. Potentially toxic peptides, including the amyloid beta-peptide that accumulates in Alzheimer\'s disease, are ligands for p75NTR.
REFERENCES
1. Bibel, M et al: EMBO J. 18:616-22, 1999
2. Barker, P.A.: Neuron 42:529-33, 2004
3. Mamidipudi, V. & Wooten, M.W.: J Neurosci. Res. 68:373-84, 2002
4. DeFreitas, M.F. et al: J. Neurosci. 21:5121-9, 2001
2. Barker, P.A.: Neuron 42:529-33, 2004
3. Mamidipudi, V. & Wooten, M.W.: J Neurosci. Res. 68:373-84, 2002
4. DeFreitas, M.F. et al: J. Neurosci. 21:5121-9, 2001
Products are for research use only. They are not intended for human, animal, or diagnostic applications.
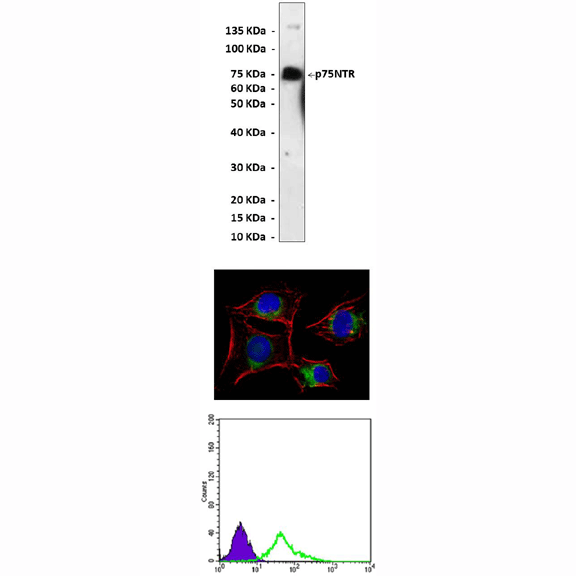
(Click to Enlarge) Top: Western Blot detection of p75NTR proteins overexpressed in 293 cells using p75NTR Antibody. Middle: This antibody stains EC cells in confocal immunofluorescent analysis (p75NTR Antibody: Green; Actin filaments: Red; DRAQ5 DNA dye: Blue). Bottom: It also specifically reacts with p75NTR proteins in EC cells in FACS testing (p75NTR Antibody: Green vs. normal mouse IgG control: Blue).
Details
Cat.No.: | CP10185 |
Antigen: | Purified recombinant human p75NTR fragments expressed in E. coli. |
Isotype: | Mouse IgG |
Species & predicted species cross- reactivity ( ): | Human |
Applications & Suggested starting dilutions:* | WB 1:1000 IP n/d IHC n/d ICC 1:200 FACS 1:200 |
Predicted Molecular Weight of protein: | 75 kDa |
Specificity/Sensitivity: | Detects endogenous p75NTR proteins without cross-reactivity with other family members. |
Storage: | Store at -20°C, 4°C for frequent use. Avoid repeated freeze-thaw cycles. |
*Optimal working dilutions must be determined by end user.
Products
| Product | Size | CAT.# | Price | Quantity |
|---|---|---|---|---|
| Mouse p75 Neurotrophin Receptor Antibody: Mouse p75 Neurotrophin Receptor Antibody | Size: 100 ul | CAT.#: CP10185 | Price: $413.00 |
