Product Sheet CP10363
Description
BACKGROUND PDK1 is a Ser/Thr protein kinase that activates at least 23 protein kinases belonging to the AGC family of protein kinases by phosphorylating their T-loops. PDK1 phosphorylated Thr308 of PKB that is required step for PKB activation. PDK1 possesses an N-terminal catalytic domain and a C-terminal PH domain. The latter binds with high affinity to the PtdIns(3,4,5)P3 and PtdIns(3,4)P2 second messengers that are generated by PI-3 kinase following stimulation of cells with growth factors. The only PDK1 AGC kinase substrates to also possess a PtdIns(3,4,5)P3/PtdIns(3,4)P2 binding PH domain are isoforms of PKB (also known as Akt). Much evidence indicates that the mutual binding of PKB and PDK1 to PtdIns(3,4,5)P3/PtdIns(3,4)P2 at the plasma membrane co-localizes these enzymes and is necessary for PKB to be activated by PDK1. In contrast, the other PDK1 AGC kinase substrates, including p70 ribosomal S6-kinase, serum- and glucocorticoid-responsive kinase, and p90 ribosomal S6 kinase, do not bind PtdIns(3,4,5)P3/PtdIns(3,4)P2 or possess PH domains. Instead, these kinases possess conserved PDK1 docking sites located in a C-terminal region known as the hydrophobic motif (HM). Growth factors and hormones lead to the phosphorylation of the hydrophobic motif of these AGC kinases, significantly enhancing their ability to interact with PDK1. The region on PDK1 that interacts with the hydrophobic motif of its substrates is termed the HM-pocket and is located on the N-terminal smaller lobe of the catalytic domain.1
PDK1 seems to exist in an active, phosphorylated configuration under basal conditions and appears to be refractive to additional activation and phosphorylation upon cell stimulation with agonists which activate PI-3 kinase. PDK1 itself is also an AGC kinase member and, like its substrates, requires to be phosphorylated at its T-loop (Ser-241) to be activated. In vivo, PDK1 is capable of autophosphorylation at Ser-241 by an intermolecular reaction and is thus constitutively phosphorylated at Ser-241. The structural analysis of the PDK1 kinase domain has revealed that, similar to what has been observed in other kinases, the phosphorylated Ser-241 residue forms key interactions coordinating and aligning important catalytic motifs such as the alphaC-helix of the N-terminal lobe.2 The alphaC-helix plays a key role in all kinases as it contributes crucial residues to coordinating ATP. More recently the structure of PKBbeta in the unphosphorylated, inactive state was reported to possess a completely disordered alphaC-helix. PDK1-mediated phosphorylation of PKBbeta and interaction of PKBbeta with its own hydrophobic motif (HM) led to ordering of this helix, resulting in PKB activation. This suggested a new regulatory alphaC-helix-dependent mechanism in PKB. PDK1 is activated through interaction with its substrates too.3 It is controversial whether PDK1 translocates to the plasma membrane in response to growth-factor stimulation. One study reported that PDK1 translocated to the membranes of endothelial cells in response to platelet-derived growth factor (PDGF). PDK1 appears to be excluded from the nucleus in both stimulated and unstimulated cells. However, it was shown that PC-3 cells expressed phosphorylated and nonphosphorylated PDK-1 in the cytoplasm and nucleoplasm. In contrast, neuronal PDK-1 was located in the nucleoplasm and the phosphorylated form was located along the perinuclear region. Furthermore, it was reported that IGF-I transiently increased phosphorylation of neuronal PDK-1, resulting in its translocation to other cellular compartments.4
PDK1 seems to exist in an active, phosphorylated configuration under basal conditions and appears to be refractive to additional activation and phosphorylation upon cell stimulation with agonists which activate PI-3 kinase. PDK1 itself is also an AGC kinase member and, like its substrates, requires to be phosphorylated at its T-loop (Ser-241) to be activated. In vivo, PDK1 is capable of autophosphorylation at Ser-241 by an intermolecular reaction and is thus constitutively phosphorylated at Ser-241. The structural analysis of the PDK1 kinase domain has revealed that, similar to what has been observed in other kinases, the phosphorylated Ser-241 residue forms key interactions coordinating and aligning important catalytic motifs such as the alphaC-helix of the N-terminal lobe.2 The alphaC-helix plays a key role in all kinases as it contributes crucial residues to coordinating ATP. More recently the structure of PKBbeta in the unphosphorylated, inactive state was reported to possess a completely disordered alphaC-helix. PDK1-mediated phosphorylation of PKBbeta and interaction of PKBbeta with its own hydrophobic motif (HM) led to ordering of this helix, resulting in PKB activation. This suggested a new regulatory alphaC-helix-dependent mechanism in PKB. PDK1 is activated through interaction with its substrates too.3 It is controversial whether PDK1 translocates to the plasma membrane in response to growth-factor stimulation. One study reported that PDK1 translocated to the membranes of endothelial cells in response to platelet-derived growth factor (PDGF). PDK1 appears to be excluded from the nucleus in both stimulated and unstimulated cells. However, it was shown that PC-3 cells expressed phosphorylated and nonphosphorylated PDK-1 in the cytoplasm and nucleoplasm. In contrast, neuronal PDK-1 was located in the nucleoplasm and the phosphorylated form was located along the perinuclear region. Furthermore, it was reported that IGF-I transiently increased phosphorylation of neuronal PDK-1, resulting in its translocation to other cellular compartments.4
REFERENCES
1. Mora, A. et al: Semin. Cell Dev. Biol. 15:161-70, 2004
2. Casamayor, A. et al: Biochem. J. 342(Pt.2):287-92, 1999
3. Komander, D. et al: EMBO J. 23:3918-28, 2004
4. Alajajian, B,P. et al: Neuroreport 20:579-83, 2009
2. Casamayor, A. et al: Biochem. J. 342(Pt.2):287-92, 1999
3. Komander, D. et al: EMBO J. 23:3918-28, 2004
4. Alajajian, B,P. et al: Neuroreport 20:579-83, 2009
Products are for research use only. They are not intended for human, animal, or diagnostic applications.
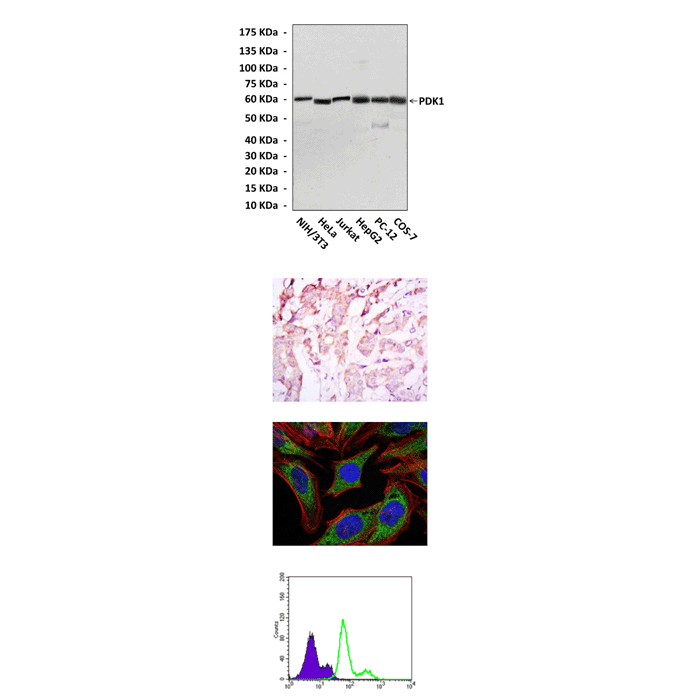
(Click to Enlarge) Top: Western blot detection of PDK1 proteins in various cell lysates using PDK1 Antibody. Middle upper: This antibody stains paraffin-embedded human breast cancer tissue in immunohistochemical analysis. Middle lower: It also stains HeLa cells in confocal immunofluorescent assay (PDK1 antibody: Green; Actin filaments: Red; DRAQ5 DNA Dye: Blue). Bottom: This antibody specifically was shown to react with PDK1 proteins in Lovo cells by FACS (PDK1 Antibody: Green; control mouse IgG: purple)
Details
Cat.No.: | CP10363 |
Antigen: | Raised against recombinant human PDK1 fragments expressed in E. coli. |
Isotype: | Mouse IgG1 |
Species & predicted species cross- reactivity ( ): | Human, Mouse, Rat |
Applications & Suggested starting dilutions:* | WB 1:1000 IP n/d IHC 1:50 - 1:200 ICC 1:50 - 1:200 FACS 1:50 - 1:200 |
Predicted Molecular Weight of protein: | 58-68 kDa |
Specificity/Sensitivity: | Detects PDK1 proteins in various cell lysate. |
Storage: | Store at -20°C, 4°C for frequent use. Avoid repeated freeze-thaw cycles. |
*Optimal working dilutions must be determined by end user.
Products
| Product | Size | CAT.# | Price | Quantity |
|---|---|---|---|---|
| Mouse PDK1 Antibody: Mouse PDK1 Antibody | Size: 100 ul | CAT.#: CP10363 | Price: $457.00 |
