Product Sheet CP10424
Description
BACKGROUND Desmosomes are a type of cadherin-based intercellular junction crucial for structural stability of tissues during embryonic development and adult tissue morphogenesis. In addition to its roles in maintaining tissue integrity, evidence is emerging that desmosomes also participate in signal transduction and transcriptional regulation. Plakoglobin (PG; also known as junction plakoglobin, JUP) is a member of the Armadillo family of proteins, and a close relative of β-catenin. Plakoglobin is common to the intracellular plaques of adhesive junctions, predominantly desmosomes and adherens junctions. It is able to associate with the cytoplasmic tails of both classic and desmosomal cadherins (classical cadherins are functionally linked to the actin-based cytoskeleton, and desmosomal cadherins, in contrast, are linked to the intermediate filament system). In desmosomes, PG participates in linking the cytoplasmic tail of desmosomal cadherins to intermediate filaments. Accordingly, PG plays a critical role in desmosomal integrity. Moreover, it was shown that PG is capable of regulating single cell motility through matrix deposition in concert with Rho GTPases, independently of its role as a cell–cell adhesion molecule.1 PG is also found in the cytoplasm and nucleus, where it is able to act independently of its function in intercellular adhesion. Its adhesion-independent functions are still not well defined, but the data suggest that PG can regulate gene expression and protein stability in both a β-catenin-dependent and -independent manner. It was shown that PG can also bind to TCF/LEF and either activate or suppress gene transcription, depending on the targets and cellular context. For example, PG was shown to suppress the expression of c-Myc in epidermal keratinocytes in a Lef-1-dependent manner.2
PG and the homologous protein β-catenin share 88% amino acid identity and bind to common protein partners; therefore, they functionally compete with each other. The primary structures of plakoglobin and β-catenin consist of a central armadillo repeat (arm) domain, flanked by the N- and C-terminal “tails”. The tails appear to be unstructured, as they are highly sensitive to proteolysis both in β-catenin and plakoglobin. Moreover, the sequences of these regions are predicted to be intrinsically disordered. The sequences of the arm domains of these two proteins are 79% identical, whereas the N- and C-terminal tails display lower identities of 45 and 27%, respectively. The arm domain of β-catenin (βcat-arm) is an elongated structure of 12 arm repeats, each of which consists of three α-helices. Successive arm repeats pack together to form a superhelical structure that features a 95-Å long positively charged groove. The groove proves to be the binding site of many partners, including cadherins, Tcf family transcription factors, the adenomatous polyposis coli tumor suppressor Axin, and the transcriptional inhibitor ICAT. No structural information has been available for plakoglobin, but its high sequence identity with β-catenin arm domains suggests that the two structures are very similar, including their binding sites for cadherins and other ligands.3
PG and the homologous protein β-catenin share 88% amino acid identity and bind to common protein partners; therefore, they functionally compete with each other. The primary structures of plakoglobin and β-catenin consist of a central armadillo repeat (arm) domain, flanked by the N- and C-terminal “tails”. The tails appear to be unstructured, as they are highly sensitive to proteolysis both in β-catenin and plakoglobin. Moreover, the sequences of these regions are predicted to be intrinsically disordered. The sequences of the arm domains of these two proteins are 79% identical, whereas the N- and C-terminal tails display lower identities of 45 and 27%, respectively. The arm domain of β-catenin (βcat-arm) is an elongated structure of 12 arm repeats, each of which consists of three α-helices. Successive arm repeats pack together to form a superhelical structure that features a 95-Å long positively charged groove. The groove proves to be the binding site of many partners, including cadherins, Tcf family transcription factors, the adenomatous polyposis coli tumor suppressor Axin, and the transcriptional inhibitor ICAT. No structural information has been available for plakoglobin, but its high sequence identity with β-catenin arm domains suggests that the two structures are very similar, including their binding sites for cadherins and other ligands.3
REFERENCES
1. Todorović, V. et al: J. Cell Sci. 123:3576-86, 2010
2. Li, J. et al: Mol. Cell. Biol. 31:1134-44, 2011
3. Choi, H.J. et al: J. Biol. Chem. 284:31776-88, 2009
2. Li, J. et al: Mol. Cell. Biol. 31:1134-44, 2011
3. Choi, H.J. et al: J. Biol. Chem. 284:31776-88, 2009
Products are for research use only. They are not intended for human, animal, or diagnostic applications.
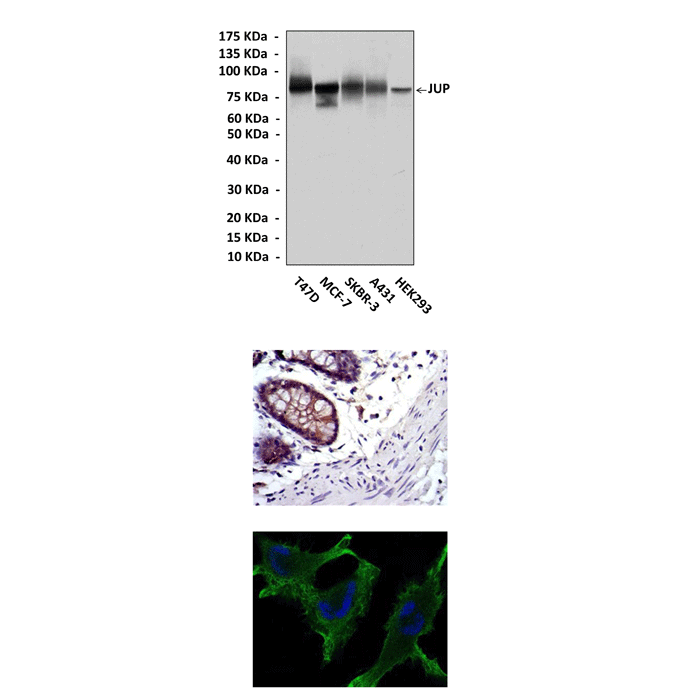
(Click to Enlarge) Top: Western blot detection of Plakoglobin/JUP proteins in various cell lysates using Plakoglobin/JUP Antibody. Middle: This antibody stains paraffin-embedded human rectum tissue in IHC analysis. Bottom: It also stains U251 cells in confocal immunofluorescent assay (Plakoglobin/JUP Antibody: Green; DRAQ DNA Dye: Blue)
Details
Cat.No.: | CP10424 |
Antigen: | Raised against recombinant human Plakoglobin fragments expressed in E. coli. |
Isotype: | Mouse IgG |
Species & predicted species cross- reactivity ( ): | Human, Mouse |
Applications & Suggested starting dilutions:* | WB 1:1000 IP n/d IHC 1:50 - 1:200 ICC 1:50 - 1:200 FACS n/d |
Predicted Molecular Weight of protein: | 82 kDa |
Specificity/Sensitivity: | Detects Plakoglobin proteins in various cell lysate. |
Storage: | Store at -20°C, 4°C for frequent use. Avoid repeated freeze-thaw cycles. |
*Optimal working dilutions must be determined by end user.
Products
| Product | Size | CAT.# | Price | Quantity |
|---|---|---|---|---|
| Mouse Plakoglobin/JUP Antibody: Mouse Plakoglobin/JUP Antibody | Size: 100 ul | CAT.#: CP10424 | Price: $457.00 |
