Product Sheet CP10211
Description
BACKGROUND The Rat Sarcoma (Ras) oncoprotein small GTPase superfamily contains over 170 members, divided into five subfamilies—Ras, Rho, Rab, Ran and Arf. Rab proteins comprise the largest subfamily of small GTPases, with more than 70 putative members in the human genome. Members of the Rab superfamily play important roles in regulating signal transduction and, subsequently, a diverse range of cellular processes, including differentiation, proliferation, intracellular vesicular transport and trafficking of proteins between organelles of the endocytic and secretory pathways, nuclear assembly and cytoskeleton formation.1
Rab25 (also known as CATX-8) is a member of the Ras-associated binding (Rab) family of small GTPases. It functions as molecular switche regulating vesicular transport in eukaryotes cells. In contrast to most Rabs, which are ubiquitously expressed, Rab25 expression was confined to the gastrointestinal mucosa, lung and kidney: the highest levels of expression were in the colon and ileal epithelium. The ubiquitously expressed Rab11 proteins (Rab11a and Rab11b) are the closest homologues to Rab25, forming the Rab11 subfamily. Rab11 subfamily proteins, like all Ras superfamily proteins, are thought to share a conserved mechanism of regulation. The activity of the protein is determined by the relative amount of GTP-bound (active) versus GDP-bound (inactive) forms. GTP binding induces conformational changes in the switch I and switch II regions, resulting in the modulation of binding affinities that are critical for association with regulatory and effector proteins. In vivo, the GDP/GTP exchange and GTPase activity are regulated by a complex regulatory network consisting of several classes of proteins, including guanine nucleotide exchange factors, which promote dissociation of bound GDP and formation of the active GTP-bound complex, whereas GTPase-activating proteins accelerate the intrinsic GTPase activity of the small GTPases to promote formation of the inactive GDP-bound form. Rab GTPases are further regulated by guanine nucleotide dissociation inhibitors that inhibit GDP dissociation and promote cytosolic sequestration of these GTPases. Rab11 and Rab25, detected in the apical recycling endosome (ARE), perinuclear recycling endosome (PRE) and trans Golgi network (TGN). Once activated, Rab11 and Rab25 recruit specific downstream effectors for different biological functions.2 In addition, Rab subfamily proteins terminate in a distinct set of cysteine-containing C-terminal motifs, allowing modification by geranylgeranyltransferase II that targets Rab GTPases to different membrane locations and enabling additional mechanisms of functional regulation.3
Rab25 has been implicated in the pathophysiology of ovarian, breast and other cancers. Its role in endosomal transport and recycling of cell-surface receptors and signaling proteins presents a novel paradigm for the disruption of cellular pathways and promotion of tumor development and aggressiveness. Rab25 is a determinant of tumor progression and aggressiveness of epithelial cancers. Rab25 associates with alpha5beta1 integrin, and enhances tumor cell invasion by directing the localization of integrin-containing vesicles to the leading edge of matrix invading pseudopodia.4
Rab25 (also known as CATX-8) is a member of the Ras-associated binding (Rab) family of small GTPases. It functions as molecular switche regulating vesicular transport in eukaryotes cells. In contrast to most Rabs, which are ubiquitously expressed, Rab25 expression was confined to the gastrointestinal mucosa, lung and kidney: the highest levels of expression were in the colon and ileal epithelium. The ubiquitously expressed Rab11 proteins (Rab11a and Rab11b) are the closest homologues to Rab25, forming the Rab11 subfamily. Rab11 subfamily proteins, like all Ras superfamily proteins, are thought to share a conserved mechanism of regulation. The activity of the protein is determined by the relative amount of GTP-bound (active) versus GDP-bound (inactive) forms. GTP binding induces conformational changes in the switch I and switch II regions, resulting in the modulation of binding affinities that are critical for association with regulatory and effector proteins. In vivo, the GDP/GTP exchange and GTPase activity are regulated by a complex regulatory network consisting of several classes of proteins, including guanine nucleotide exchange factors, which promote dissociation of bound GDP and formation of the active GTP-bound complex, whereas GTPase-activating proteins accelerate the intrinsic GTPase activity of the small GTPases to promote formation of the inactive GDP-bound form. Rab GTPases are further regulated by guanine nucleotide dissociation inhibitors that inhibit GDP dissociation and promote cytosolic sequestration of these GTPases. Rab11 and Rab25, detected in the apical recycling endosome (ARE), perinuclear recycling endosome (PRE) and trans Golgi network (TGN). Once activated, Rab11 and Rab25 recruit specific downstream effectors for different biological functions.2 In addition, Rab subfamily proteins terminate in a distinct set of cysteine-containing C-terminal motifs, allowing modification by geranylgeranyltransferase II that targets Rab GTPases to different membrane locations and enabling additional mechanisms of functional regulation.3
Rab25 has been implicated in the pathophysiology of ovarian, breast and other cancers. Its role in endosomal transport and recycling of cell-surface receptors and signaling proteins presents a novel paradigm for the disruption of cellular pathways and promotion of tumor development and aggressiveness. Rab25 is a determinant of tumor progression and aggressiveness of epithelial cancers. Rab25 associates with alpha5beta1 integrin, and enhances tumor cell invasion by directing the localization of integrin-containing vesicles to the leading edge of matrix invading pseudopodia.4
REFERENCES
1. Agarwal, R. et al: Traffic 10:1561-8, 2009
2. Chia, W.J. &, Tang, B.L.: Biochim Biophys Acta. 1795:110-6, 2009
3. Tang, B.L. & Ng, E.L.: Cell Motil Cytoskeleton. 66:365-70, 2009
4. Caswell, P.T. et al: Dev. Cell 13:496-510, 2007
2. Chia, W.J. &, Tang, B.L.: Biochim Biophys Acta. 1795:110-6, 2009
3. Tang, B.L. & Ng, E.L.: Cell Motil Cytoskeleton. 66:365-70, 2009
4. Caswell, P.T. et al: Dev. Cell 13:496-510, 2007
Products are for research use only. They are not intended for human, animal, or diagnostic applications.
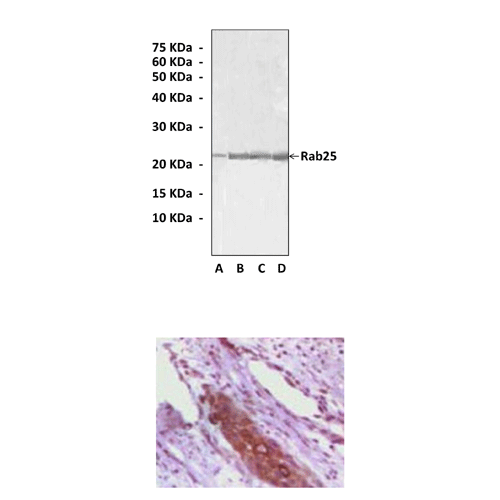
(Click to Enlarge) Top: Western Blot detection of Rab25 proteins in Rab25 recombinant Protein (A), Human Ovary Carcinoma (B), Stomach Carcinoma (C), and Breast Carcinoma (D) tissue lysates using Rab25 Antibody. Bottom: This antibody stains paraffin-embedded human skin carcinoma tissue in immunohistochemical analysis.
Details
Cat.No.: | CP10211 |
Antigen: | Purified recombinant human Gab25 proteins expressed in E. coli. |
Isotype: | Mouse IgG2a |
Species & predicted species cross- reactivity ( ): | Human |
Applications & Suggested starting dilutions:* | WB 1:1000 IP 1:50 IHC 1:200 ICC n/d FACS n/d |
Predicted Molecular Weight of protein: | 23 kDa |
Specificity/Sensitivity: | Detects endogenous Rab25 proteins without cross-reactivity with other family members. |
Storage: | Store at -20°C, 4°C for frequent use. Avoid repeated freeze-thaw cycles. |
*Optimal working dilutions must be determined by end user.
Products
| Product | Size | CAT.# | Price | Quantity |
|---|---|---|---|---|
| Mouse Ras-Associated Binding factor 25 Antibody: Mouse Ras-Associated Binding factor 25 Antibody | Size: 100 ul | CAT.#: CP10211 | Price: $531.00 |
