Product Sheet CP10392
Description
BACKGROUND The biological importance of retinoids (vitamin A and its derivatives) for vertebrate development has long been known, because both deprivation of and excessive exposure to retinoids cause major embryonic abnormalities. Vitamin A is the precursor of at least two critical metabolites: 11-cis-retinal, the chromophore of visual G protein-coupled receptors, and all-trans-retinoic acid (RA). RA regulates gene expression via heterodimeric nuclear receptors consisting of both RA receptors (RARs) and retinoid X receptors (RXRs). Both are ligand-dependent transcription factors belonging to the superfamily of nuclear hormone receptors. A prerequisite for initiation of retinoid-dependent physiological processes is the coordinated production of biologically active retinoids from circulating precursors. To achieve this, cells and tissues must be adequately supplied with this vitamin throughout their life cycle. All-trans-retinol bound to the retinol-binding protein RBP4 (holo-RBP4) serves as the major transport mode for vitamin A in the blood. RBP4 is the only specific transport protein for retinol (vitamin A) in the circulation and its main function is thought to be the delivery of retinol to tissues. It has long been postulated that cellular retinol uptake from holo-RBP4 is a facilitated, protein-mediated process. Recently, it has been suggested that the multitransmembrane domain protein STRA6 acts as the long-sought RBP4 receptor. In cell cultures, STRA6 specifically binds to holo-RBP4 and mediates the cellular uptake of retinol. In mammals, STRA6 is expressed in a variety of embryonic and adult cells and tissues.1
RBP4 is now identified as an new adipocytokine that has been associated with insulin resistance. Both RBP4 mRNA expression in adipocytes and serum RBP4 levels are elevated in adipose-specific glucose transporter 4-knockout mice, and elevated circulating RBP4 levels cause insulin resistance by inhibiting phosphatidylinositol 3 kinase activity in skeletal muscle and increasing phosphoenolpyruvate carboxylase expression in liver.2 Several clinical cross-sectional studies have shown a significant negative association between circulating RBP4 levels and insulin sensitivity evaluated by the glucose clamp method, but it is unclear if RBP4 is associated with insulin resistance in humans because of many conflicting results. Drugs such as rosiglitazone, exercise and weight loss have been shown to decrease circulating RBP4 levels and improve insulin resistance, but contradictory results have been found in other studies. In addition, a recent clinical study has suggested that RBP4 is more closely related to visceral adiposity than subcutaneous adiposity. Thus, whether or not RBP4 is a new target for treatment of Type 2 diabetes remains to be determined. However, growing evidence suggests that RBP4 may play more important role in lipid metabolism rather than insulin resistance. For example, most of the previous human studies that confirmed the association of RBP4 levels with insulin resistance also observed significant associations with lipid levels, in particular with triglyceride, high-density lipoprotein (HDL)-cholesterol and low-density lipoprotein (LDL)-cholesterol. Others only observed associations of RBP4 with increased triglycerides levels and with pro-atherogenic lipoproteins or key enzymes of lipoprotein metabolism, but not with insulin resistance marker.2 Since hypertriglyceridemia plays an important role in the pathogenesis of cardiovascular disease, circulating RBP4 levels might emerge as a suitable target for therapeutic intervention in cardiovascular disease. In addition, it was shown that hepatic storage of RBP4, unrelated to its expression, could cause liver damage both in chronic hepatitis C (CHC) and nonalcoholic steatohepatitis (NASH) patients.3
RBP4 is now identified as an new adipocytokine that has been associated with insulin resistance. Both RBP4 mRNA expression in adipocytes and serum RBP4 levels are elevated in adipose-specific glucose transporter 4-knockout mice, and elevated circulating RBP4 levels cause insulin resistance by inhibiting phosphatidylinositol 3 kinase activity in skeletal muscle and increasing phosphoenolpyruvate carboxylase expression in liver.2 Several clinical cross-sectional studies have shown a significant negative association between circulating RBP4 levels and insulin sensitivity evaluated by the glucose clamp method, but it is unclear if RBP4 is associated with insulin resistance in humans because of many conflicting results. Drugs such as rosiglitazone, exercise and weight loss have been shown to decrease circulating RBP4 levels and improve insulin resistance, but contradictory results have been found in other studies. In addition, a recent clinical study has suggested that RBP4 is more closely related to visceral adiposity than subcutaneous adiposity. Thus, whether or not RBP4 is a new target for treatment of Type 2 diabetes remains to be determined. However, growing evidence suggests that RBP4 may play more important role in lipid metabolism rather than insulin resistance. For example, most of the previous human studies that confirmed the association of RBP4 levels with insulin resistance also observed significant associations with lipid levels, in particular with triglyceride, high-density lipoprotein (HDL)-cholesterol and low-density lipoprotein (LDL)-cholesterol. Others only observed associations of RBP4 with increased triglycerides levels and with pro-atherogenic lipoproteins or key enzymes of lipoprotein metabolism, but not with insulin resistance marker.2 Since hypertriglyceridemia plays an important role in the pathogenesis of cardiovascular disease, circulating RBP4 levels might emerge as a suitable target for therapeutic intervention in cardiovascular disease. In addition, it was shown that hepatic storage of RBP4, unrelated to its expression, could cause liver damage both in chronic hepatitis C (CHC) and nonalcoholic steatohepatitis (NASH) patients.3
REFERENCES
1. von Eynatten, M. & Humpert, P.M.: Expert Rev. Mol. Diagn. 8:289-99, 2008
2. Wu, Y. et al: J. Lipid Res.50:1479-86, 2009
3. Petta, S. et al: Digest. Liver Dis. 43:404-10, 2011
2. Wu, Y. et al: J. Lipid Res.50:1479-86, 2009
3. Petta, S. et al: Digest. Liver Dis. 43:404-10, 2011
Products are for research use only. They are not intended for human, animal, or diagnostic applications.
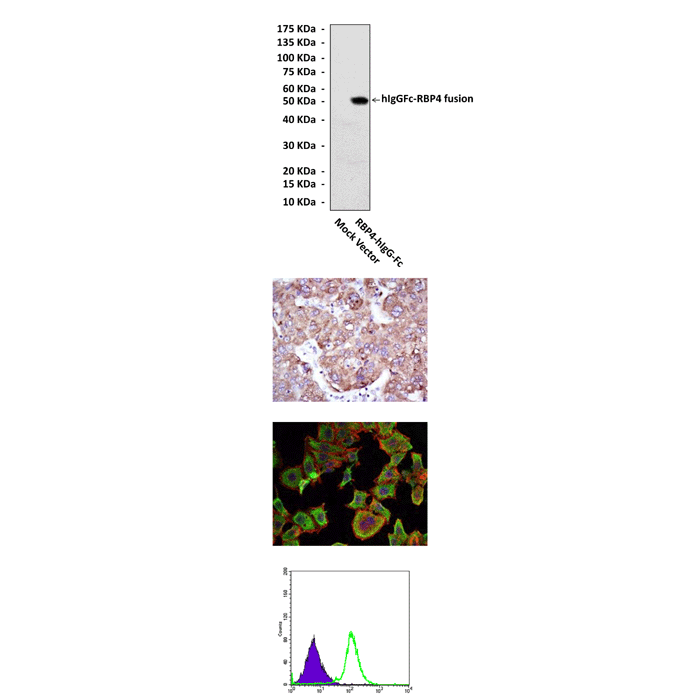
(Click to Enlarge) Top: Western blot detection of RBP4 proteins in 293 cell lysates transfected with human RBP4-hIgG-Fc fusion protein expression vector or mock vector using RBP4 Antibody. Middle Upper: This antibody stains paraffin-embedded human liver cancer tissue in IHC analysis. Middle Lower: It also stains HepG2 cells in confocal immunofluorescent testing (RBP4 Antibody: Green; Actin filaments: Red; DRAQ5 DNA Dye: Blue). Bottom: This antibody detects RBP4 proteins specifically in HepG2 cells by FACS assay (RBP4 Antibody: Green; negative control: Purple).
Details
Cat.No.: | CP10392 |
Antigen: | Raised against recombinant human RBP4 fragments expressed in E. coli. |
Isotype: | Mouse IgG1 |
Species & predicted species cross- reactivity ( ): | Human |
Applications & Suggested starting dilutions:* | WB 1:1000 IP n/d IHC 1:50 - 1:200 ICC 1:50 - 1:200 FACS 1:50 - 1:200 |
Predicted Molecular Weight of protein: | 23 kDa |
Specificity/Sensitivity: | Detects RBP4 proteins in various cell lysate. |
Storage: | Store at -20°C, 4°C for frequent use. Avoid repeated freeze-thaw cycles. |
*Optimal working dilutions must be determined by end user.
Products
| Product | Size | CAT.# | Price | Quantity |
|---|---|---|---|---|
| Mouse RBP4 Antibody: Mouse RBP4 Antibody | Size: 100 ul | CAT.#: CP10392 | Price: $457.00 |
