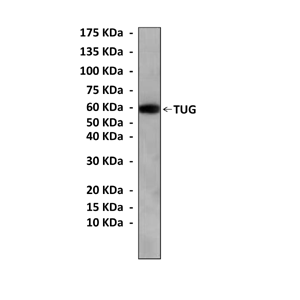
Anti-TUG: Mouse TUG Antibody
Mouse TUG Antibody: Mouse TUG Antibody
Size: 100 ul
Price: $354.00
Description
In unstimulated cells, GLUT4 that is present in GSVs may be tethered to intracellular structures to restrict its movement to the plasma membrane. Insulin “untethers” this GLUT4 to accelerate its movement to the cell surface and subsequent fusion at the plasma membrane. TUG (tether, containing a UBX domain, for GLUT4) is a putative tethering protein, which acts in concert with other proteins to retain GLUT4 within cells in the absence of insulin.2 As a cytosolic protein, TUG binds directly and specifically to GLUT4 and not GLUT1. TUG and GLUT4 form a complex in unstimulated 3T3-L1 adipocytes, and colocalize on TfnR-negative intracellular membranes that sediment as light microsomes. These are properties shared by GSVs. The TUG-GLUT4 complex is insulin-responsive, because insulin acts rapidly to stimulate the dissociation of TUG and GLUT4 and releasing these vesicles to the cellular exocytic machinery for translocation to the plasma membrane. Overexpression of TUG increases the size of the insulin-responsive GLUT4 pool, assessed using kinetics, and increases the number of insulin-responsive TUG-GLUT4 protein complexes. Conversely, expression of a dominant negative TUG UBX-Cter fragment decreases the size of the insulin-responsive pool and decreases the number of TUG-GLUT4 complexes. Thus, TUG is part of an insulin-responsive mechanism governing the sequestration of GLUT4 in GSVs within unstimulated 3T3-L1 cells. TUG is required to retain GLUT4 intracellularly in 3T3-L1 adipocytes in the absence of insulin and the insulin-stimulated dissociation of TUG and GLUT4 is an important mechanism by which insulin stimulates glucose uptake.3 TUG is also found to regulate skeletal muscle Glut4 traffic in transgenic mouse model.4
2. Bogan, J.S. et al: Nature 425:7727-33, 2003
3. Yu, C. et al: J. Biol. Chem. 282:7710-22, 2007
4. Schertzer, J. D. et al: Endocrinol. 150:1935-40, 2009
Details
| Cat.No.: | CP10241 |
| Antigen: | Purified recombinant human TUG fragments expressed in E. coli. |
| Isotype: | Mouse IgG |
| Species & predicted species cross- reactivity ( ): | Human, Mouse |
| Applications & Suggested starting dilutions:* | WB 1:1000 IP n/d IHC n/d ICC n/d FACS n/d |
| Predicted Molecular Weight of protein: | 60 kDa |
| Specificity/Sensitivity: | Detects endogenous TUG proteins without cross-reactivity with other family members. |
| Storage: | Store at -20°C, 4°C for frequent use. Avoid repeated freeze-thaw cycles. |
*Optimal working dilutions must be determined by end user.