Product Sheet CP10361
Description
BACKGROUND USP7, also known as the hepes simplex virus associated ubiquitin-specific protease (HAUSP), deubiquitinates both mdm2 and p53, and plays an important role in regulating the level and activity of p53. USP7/ HAUSP was first identified as a 135 kD cellular factor associated with the herpesvirus regulatory protein Vmw110. USP7/HAUSP is involved in the cellular stress response by regulating Mdm2 and p53 protein levels following severe DNA damage. Interestingly, reduction or ablation of USP7/HAUSP leads to the DNA-damage-induced MdmX degradation and instability of Mdm2, leading to a robust stabilization of p53. Moreover, knocking down USP7/HAUSP was shown to selectively exert antiproliferative effect through induction of cell cycle arrest. This phenotype was observed only in cancer cells retaining wild-type p53. Consistent with recent reports, USP7/HAUSP silencing has also been shown to increase steady-state p53 levels by promoting Mdm2 degradation. Furthermore, it was shown that the amino-terminal domain (USP7–NTD) of USP7/ HAUSP interacts with distinct regions on p53 and Hdm2 containing P/AxxS motifs, whereas, the C-terminal domain of USP7/ HAUSP is responsible for maintaining the active conformation for catalysis and inhibitor binding, and contains the prime side of the proteolytic active site.1 It is becoming clear that Mdm2, rather than p53, is the preferred substrate of USP7/HAUSP under normal unstressed conditions. This results in Mdm2 stabilization through antagonization of its autoubiquitination and consequently in induction of p53 degradation. Structural and biochemical data have shown that Mdm2 and p53 associate with USP7/HAUSP in a mutually exclusive manner but that USP7/HAUSP has a higher binding affinity for Mdm2. More generally, USP7/HAUSP can deubiquitinate different targets, including Mdm2 and p53, and the net deubiquitination of these latter targets ultimately determines functional p53 levels. Thus, these four proteins, p53, Mdm2, MdmX, and USP7/ HAUSP have a delicate, intimate balance that maintains p53 proteins levels.2 And because of the strong p53 induction that occurs in the absence of USP7/ HAUSP, it represents a potential chemotherapeutic target for the stabilization and activation of p53 in cells that retain a wild-type copy of the gene. USP7/HAUSP has also been shown to deubiquitinate FOXO4, provoking its nuclear export and hence its inactivation; consequently, the oncogenic phosphatidylinositol 3-kinase/protein kinase B signaling pathway was activated. The tumor suppressor PTEN is also deubiquitinated by USP7/ HAUSP, resulting in its nuclear export and inactivation. In addition to this, USP7/ HAUSP may also play a role in chromatin remodeling by direct deubiquitylation of histones, as well as indirectly by regulating the cellular levels of E3 ubiquitin ligases involved in histone ubiquitylation. It was demonstrated that USP7/HAUSP modulated chromatin remodeling is important for base excision repair of oxidative lesions.3 Additionally, Daxx is a multifunctional protein, regulating a wide range of important functions including apoptosis and transcription.It was shown that that USP7/ HAUSP critically controls the cellular level of Daxx most likely by inducing Daxx de-ubiquitination.4 USP7/ HAUSP was shown to play important role in stem cell maintenance.5 Finally, DNA methyltransferase 1 (DNMT1) is the primary enzyme that maintains DNA methylation. DNMT1 was destabilized by acetylation by the acetyltransferase Tip60, which triggered ubiquitination by the E3 ligase UHRF1, thereby targeting DNMT1 for proteasomal degradation. In contrast, DNMT1 was stabilized by histone deacetylase 1 (HDAC1) and the deubiquitinase USP7/ HAUSP.6
REFERENCES
1. Ma, J. et al: Arch. Biochem. Biophy. 503:207-12, 2010
2. Li, M. et al: Mol. Cell 13:879-86, 2004
3. Khoronenkova, S.V. et al: Nucleic Acids Res 2010 (In Press)
4. Tang, J. et al: Biochem. Biophy. Res. Commun. 393:542-5, 2010
5. Huang, Z. et al.: Nat. Cell Biol. 13:142-52, 2011
6. Du, Z. et al: Sci. Signal 2:ra80, 2010
2. Li, M. et al: Mol. Cell 13:879-86, 2004
3. Khoronenkova, S.V. et al: Nucleic Acids Res 2010 (In Press)
4. Tang, J. et al: Biochem. Biophy. Res. Commun. 393:542-5, 2010
5. Huang, Z. et al.: Nat. Cell Biol. 13:142-52, 2011
6. Du, Z. et al: Sci. Signal 2:ra80, 2010
Products are for research use only. They are not intended for human, animal, or diagnostic applications.
Details
Cat.No.: | CP10361 |
Antigen: | Raised against recombinant human USP7/ HAUSP fragments expressed in E. coli. |
Isotype: | Mouse IgG1 |
Species & predicted species cross- reactivity ( ): | Human |
Applications & Suggested starting dilutions:* | WB 1:1000 IP n/d IHC n/d ICC n/d FACS n/d |
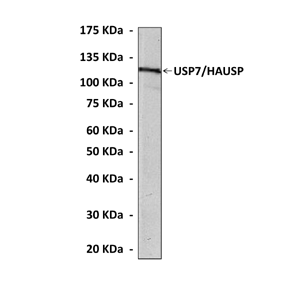
Predicted Molecular Weight of protein: | 128 kDa |
Specificity/Sensitivity: | Detects USP7/ HAUSP proteins in various cell lysate. |
Storage: | Store at -20°C, 4°C for frequent use. Avoid repeated freeze-thaw cycles. |
*Optimal working dilutions must be determined by end user.
Products
| Product | Size | CAT.# | Price | Quantity |
|---|---|---|---|---|
| Mouse USP7/HAUSP Antibody: Mouse USP7/HAUSP Antibody | Size: 100 ul | CAT.#: CP10361 | Price: $413.00 |