Product Sheet CP10244
Description
BACKGROUND The Intermediate Filament (IF) protein family is one of the largest in the human genome, with more than 65 members. Despite the limited primary sequence homology in the IF family, all cytoplasmic IF proteins are similar in predicted secondary structure, and have the ability to assemble into 8-12-nm IFs. IFs are crucial in defining key biomechanical functions of cells such as cell migration, cell division and mechanotransduction, and have also been referred to as the “safety belts of cells” to prevent exceedingly large cell stretch. IFs are also found in the cell\'s nucleus in the form of lamin, where they form a dense mesh-like network providing mechanical integrity and biochemical functions at the cytoskeleton-chromatin interface. IFs have also been associated with dozens of genetic diseases, where single point mutations and deletions lead to structural changes at different levels in the IFs organization. IF related diseases include muscle dystrophies, Alexander disease, epidermolysis bullosa simplex, as well as a broad class of disorders referred to as laminopathies (e.g. rapid aging disease progeria).1
Vimentin is a Type III IF protein, common to many cells of mesenchymal origin. The Vimentin monomer is a 53-kDa polypeptide, and like all IF proteins exhibits a conserved, predicted, tripartite domain structure, consisting of a central α-helical rod domain (∼310 amino acids long), flanked by non α-helical N- and C-terminal head and tail domains. Vimentin polymerizes via complex lateral interactions of coiled-coil dimers into long, flexible filaments. Vimentin is responsible for maintaining cell shape, integrity of the cytoplasm, and stabilizing cytoskeletal interactions. It is also involved in the immune response, and controls the transport of low-density lipoprotein (LDL)-derived cholesterol from a lysosome to the site of esterification. It functions as an organizer of a number of critical proteins involved in attachment, migration, and cell signaling. Phosphorylation has been shown to contribute to the regulation of Vimentin filament assembly and disassembly. Phosphorylation occurs exclusively in the head and tail domains, with nearly twenty phosphorylation sites identified, most of which are in the head domain. Several different kinases have been implicated, including Aurora-B and Rho-kinase, Cdk1, and Plk1 etc. Plk1 phosphorylates Vimentin at Ser82, which partly inhibits the filament forming ability of Vimentin.2 Furthermore, it was reported that Ser25, Ser38, Ser50, Ser65 and Ser72 in the amino-terminal head domain are major phosphorylation sites on Vimentin for PAK. The Vimentin phosphorylation is not only involved in regulating the assembly of Ifs, but also affect its binding with kinases.3 In addition, It was found that Vimentin participates in regulation of cell surface signaling. Studies confirmed a role of Vimentin on the cell surface for the activation of latent transforming growth factor-beta.4
Finally, six major classes of IFs are believed to be relatively specific for certain cell types, for example keratin in epithelial cells, neurofilaments in neurons, glial fibrillary acid protein in glial cells, desmin in muscle cells and Vimentin in mesenchymal cells. Obviously, they are variably expressed in different cell types and in corresponding tumors. Expression of Vimentin and cytokeratins has also been described in breast carcinomas. Moreover, Vimentin is selectively expressed in aggressive breast cancer cell lines. Elevated Vimentin expression level correlates well with up-regulated migration and invasion of cancer cells.5
Vimentin is a Type III IF protein, common to many cells of mesenchymal origin. The Vimentin monomer is a 53-kDa polypeptide, and like all IF proteins exhibits a conserved, predicted, tripartite domain structure, consisting of a central α-helical rod domain (∼310 amino acids long), flanked by non α-helical N- and C-terminal head and tail domains. Vimentin polymerizes via complex lateral interactions of coiled-coil dimers into long, flexible filaments. Vimentin is responsible for maintaining cell shape, integrity of the cytoplasm, and stabilizing cytoskeletal interactions. It is also involved in the immune response, and controls the transport of low-density lipoprotein (LDL)-derived cholesterol from a lysosome to the site of esterification. It functions as an organizer of a number of critical proteins involved in attachment, migration, and cell signaling. Phosphorylation has been shown to contribute to the regulation of Vimentin filament assembly and disassembly. Phosphorylation occurs exclusively in the head and tail domains, with nearly twenty phosphorylation sites identified, most of which are in the head domain. Several different kinases have been implicated, including Aurora-B and Rho-kinase, Cdk1, and Plk1 etc. Plk1 phosphorylates Vimentin at Ser82, which partly inhibits the filament forming ability of Vimentin.2 Furthermore, it was reported that Ser25, Ser38, Ser50, Ser65 and Ser72 in the amino-terminal head domain are major phosphorylation sites on Vimentin for PAK. The Vimentin phosphorylation is not only involved in regulating the assembly of Ifs, but also affect its binding with kinases.3 In addition, It was found that Vimentin participates in regulation of cell surface signaling. Studies confirmed a role of Vimentin on the cell surface for the activation of latent transforming growth factor-beta.4
Finally, six major classes of IFs are believed to be relatively specific for certain cell types, for example keratin in epithelial cells, neurofilaments in neurons, glial fibrillary acid protein in glial cells, desmin in muscle cells and Vimentin in mesenchymal cells. Obviously, they are variably expressed in different cell types and in corresponding tumors. Expression of Vimentin and cytokeratins has also been described in breast carcinomas. Moreover, Vimentin is selectively expressed in aggressive breast cancer cell lines. Elevated Vimentin expression level correlates well with up-regulated migration and invasion of cancer cells.5
REFERENCES
1. Herrmann, H. & Aebi, U.: Annu. Rev. Biochem. 73:749-89, 2004
2. Yamaguchi, T. et al: J. Cell Bioli. 171:431-6, 2005
3. Sin, W. et al: Mol. Cell. Biol. 18:6325-39, 1998
4. Yasutake, N. et al: FEBS Lett. 583:308-12, 2009
5. Kusinska, R.U. et al: J Exp Clin Cancer Res. 28:118, 2009
2. Yamaguchi, T. et al: J. Cell Bioli. 171:431-6, 2005
3. Sin, W. et al: Mol. Cell. Biol. 18:6325-39, 1998
4. Yasutake, N. et al: FEBS Lett. 583:308-12, 2009
5. Kusinska, R.U. et al: J Exp Clin Cancer Res. 28:118, 2009
Products are for research use only. They are not intended for human, animal, or diagnostic applications.
Details
Cat.No.: | CP10244 |
Antigen: | Purified recombinant human Vimentin fragments expressed in E. coli. |
Isotype: | Mouse IgG1 |
Species & predicted species cross- reactivity ( ): | Human, Mouse, Rat |
Applications & Suggested starting dilutions:* | WB 1:1000 - 1:5000 IP n/d IHC 1:250 - 1:500 ICC n/d FACS n/d |
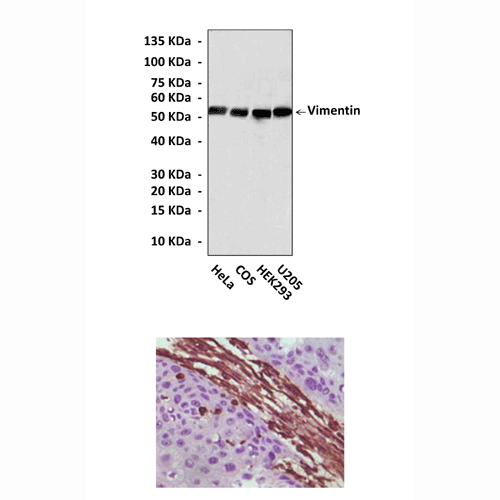
Predicted Molecular Weight of protein: | 53 kDa |
Specificity/Sensitivity: | Detects endogenous Vimentin proteins without cross-reactivity with other family members. |
Storage: | Store at -20°C, 4°C for frequent use. Avoid repeated freeze-thaw cycles. |
*Optimal working dilutions must be determined by end user.
Products
| Product | Size | CAT.# | Price | Quantity |
|---|---|---|---|---|
| Mouse Vimentin Antibody: Mouse Vimentin Antibody | Size: 100 ul | CAT.#: CP10244 | Price: $413.00 |