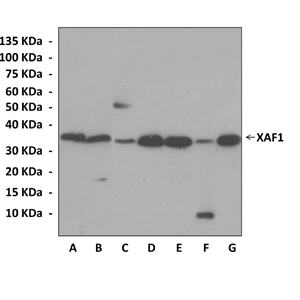
Product Sheet CA1218
Description
BACKGROUND Inhibitor of apoptosis (IAPs) proteins belong to an important antiapoptotic protein family that play central role in carcinogenesis and resistance to cancer therapy. IAPs can bind and inactivate key caspases involved in the initiation (caspase 9) and execution (caspase 3 and 7) of apoptosis cascade. Thus, IAPs represent critical regulatory factors in mitochondrial apoptosis signaling. Moreover, some IAPs such as survivin can also interact with microtubule, counteract a default induction of apoptosis in G2/M phase and favor aberrant progression of transformed cells through mitosis. X-linked inhibitor of apoptosis (XIAP) is a potent member of IAPs. Its antiapoptotic activity can be negatively regulated by an interacting protein, XAF1 (X-linked inhibitor of apoptosis-associated Factor 1).1 In addition to antagonize the effect of XIAP, accumulated evidences have implicated XAF1 as a tumor suppressor that functions through XIAP-independent pathways. The expression of XAF1 was higher in normal tissues comparing with matched cancer tissues due to promoter hypermethylation in cancer cells, while overexpression of XAF1 suppressed cancer cell growth. It was reported that XAF1 mediated all-trans retinoic acid-induced apoptosis and increased the sensitivity of cancer cell to other apoptotic triggers such as etoposide. Moreover, it was also shown that XAF1 is a novel interferon-stimulating gene that augmented TNF-related apoptosis-inducing ligand-induced apoptosis in an XIAP-independent manner. Moreover, studies confirmed that XAF1 was able to bind to other IAP proteins, including cIAP1, cIAP2, Livin, TsIAP and NAIP but not Survivin, while overexpressed XAF1 downregulates survivin protein expression.2
Several regulatory pathways of XAF1 expression have been reported. In addition to hypermethylation of XAF1 promoter, it has been demonstrated that heat shock factor 1 negatively while interferon regulatory factor-1 and STAT1 positively modulated XAF1 transcription.3 These facts implicated that XAF1 might play important role in multiple cellular events such as growth, apoptosis and stress response. Cell division or proliferation is well controlled by some key checkpoint proteins. In normal cells, G2/M checkpoint, which is usually activated by DNA damage, involves a complex network of genes involved in cell cycle arrest, DNA repair and apoptosis. Checkpoint kinase 1 (Chk1) and Chk2 are two checkpoint proteins during G2–M transition and both can phosphorylate Cdc25C to create a binding site for 14-3-3 proteins, which in turn sequester Cdc25C in the cytoplasm, preventing Cdc25 from activating Cdc2–cyclin B complex in the nucleus. Some tumor suppressor has been reported to be able to interact with and modulate the function of Chks. It was showed that XAF1 protein expression was G2/M phase dependent. It interacted with and activated Chk1 and modulated G2/M checkpoint. Overexpression of XAF1 induced cell cycle arrest at G2/M phase and resulted in mitotic catastrophe. Thus XAF1 acts as a novel cell cycle modulator and plays important role in carcinogenesis.4
Several regulatory pathways of XAF1 expression have been reported. In addition to hypermethylation of XAF1 promoter, it has been demonstrated that heat shock factor 1 negatively while interferon regulatory factor-1 and STAT1 positively modulated XAF1 transcription.3 These facts implicated that XAF1 might play important role in multiple cellular events such as growth, apoptosis and stress response. Cell division or proliferation is well controlled by some key checkpoint proteins. In normal cells, G2/M checkpoint, which is usually activated by DNA damage, involves a complex network of genes involved in cell cycle arrest, DNA repair and apoptosis. Checkpoint kinase 1 (Chk1) and Chk2 are two checkpoint proteins during G2–M transition and both can phosphorylate Cdc25C to create a binding site for 14-3-3 proteins, which in turn sequester Cdc25C in the cytoplasm, preventing Cdc25 from activating Cdc2–cyclin B complex in the nucleus. Some tumor suppressor has been reported to be able to interact with and modulate the function of Chks. It was showed that XAF1 protein expression was G2/M phase dependent. It interacted with and activated Chk1 and modulated G2/M checkpoint. Overexpression of XAF1 induced cell cycle arrest at G2/M phase and resulted in mitotic catastrophe. Thus XAF1 acts as a novel cell cycle modulator and plays important role in carcinogenesis.4
REFERENCES
1. Fong, W.G. et al: Genomics 70:113-22, 2000
2. Arora, V. et al: J. Biol. Chem. 282:26202-9, 2007
3. Sun, Y. et al: Cancer Lett.260:62-71, 2008
4. Wang, J. et al: Carcinogenesis 30:1507-16, 2009
2. Arora, V. et al: J. Biol. Chem. 282:26202-9, 2007
3. Sun, Y. et al: Cancer Lett.260:62-71, 2008
4. Wang, J. et al: Carcinogenesis 30:1507-16, 2009
Products are for research use only. They are not intended for human, animal, or diagnostic applications.
Details
Cat.No.: | CA1218 |
Antigen: | Short peptide from human XAF1sequence. |
Isotype: | Rabbit IgG |
Species & predicted species cross- reactivity ( ): | Human, Rat |
Applications & Suggested starting dilutions:* | WB 1:1000 IP n/d IHC 1:50 - 1:200 ICC n/d FACS n/d |
Predicted Molecular Weight of protein: | 36 kDa |
Specificity/Sensitivity: | Detects endogenous levels of XAF1 proteins without cross-reactivity with other related proteins. |
Storage: | Store at -20°C, 4°C for frequent use. Avoid repeated freeze-thaw cycles. |
*Optimal working dilutions must be determined by end user.
Products
| Product | Size | CAT.# | Price | Quantity |
|---|---|---|---|---|
| Rabbit X-Linked Inhibitor of Apoptosis-Associated Factor 1 Antibody: Rabbit X-Linked Inhibitor of Apoptosis-Associated Factor 1 Antibody | Size: 100 ul | CAT.#: CA1218 | Price: $375.00 |