Product Sheet CP10248
Description
BACKGROUND The Src family kinases (SFKs) have nine known members (Blk, c-Fgr, Fyn, Hck, Lck, Lyn, c-Src, c-Yes and Yrk), each encoding a cytoplasmic protein-tyrosine kinase (PTK) believed to be involved in signal transduction. Further division of the SFKs into groups A and B has been suggested based on their tissue expression profiles. Group A includes Src, Fyn, Hck, and Yes, which are expressed ubiquitously, although with various levels in different cell types. In contrast, SFK group B, consisting of Hck, Fgr, Lck, Blk, and Lyn, is expressed primarily in hematopoietic cells. Members of the Src kinase family have a very similar domain structure with a high degree of homology in the SH1 (catalytic), linker, SH2 (p-Tyr binding), SH3 (protein-protein interaction) and SH4 (membrane association) domains.1 c-Src, Fyn and Yes are ubiquitously expressed, although high levels of c-Src are found in platelets, neural tissue and osteoclasts. For c-Src, autophosphorylation of Tyr416 and dephosphorylation of Tyr527 is required to switch the kinase from the inactive closed formation to the active open formation. c-Src can be inactivated by two kinases, c-Src kinase (CSK) and CSK homologous kinase (CHK), both of which phosphorylate Tyr527 of c-Src. The activity of the Src kinase family can also be regulated by phosphatases (e.g. SHP1), binding to adaptor proteins (e.g. Cbp) and proteasomal degradation. Src kinases are key upstream mediators of both the PI3-K and MAPK signaling pathways, and have been shown to have important roles in cell proliferation, migration and survival.2
The c-yes proto-oncogene, the cellular counterpart of its viral form, v-yes (originally found in the genomes of two avian retroviruses), encodes a nonreceptor-type protein tyrosine kinase. The c-Yes protein is highly homologous to the c-Src protein tyrosine kinase: their C-terminal halves encode the kinase domains, which share 90% amino acid sequence identity, and their N-terminal SH2 and SH3 domains share 84% identity. c-Yes expression is high in mammalian neuronal cells and epithelial cells, particularly in basal keratinocytes. The expression of c-Yes appears to be modulated during keratinocyte differentiation and carcinogenesis.3 The kinase activity of c-Src is known to be negatively regulated by phosphorylation of its C-terminal tyrosine 527 residue, whose surrounding sequence is well conserved in c-Yes. Given the high homology between these two kinases and their widely overlapping tissue distributions, it is of little surprise that they are capable of performing redundant functions. c-Src and c-Yes are both activated downstream of a multitude of cell surface receptors, including receptor tyrosine kinases, G-protein-coupled receptors, and cytokine receptors. Additionally, both kinases are activated during the cell cycle transition from G2 to M phase. Despite the evidence for functional overlap, several studies have also indicated specificity between c-Src and c-Yes. The two kinases differ in their sub-cellular localization, intermolecular binding partners, activation in response to cellular stimulation, and ability to mediate downstream signaling.4
The c-yes proto-oncogene, the cellular counterpart of its viral form, v-yes (originally found in the genomes of two avian retroviruses), encodes a nonreceptor-type protein tyrosine kinase. The c-Yes protein is highly homologous to the c-Src protein tyrosine kinase: their C-terminal halves encode the kinase domains, which share 90% amino acid sequence identity, and their N-terminal SH2 and SH3 domains share 84% identity. c-Yes expression is high in mammalian neuronal cells and epithelial cells, particularly in basal keratinocytes. The expression of c-Yes appears to be modulated during keratinocyte differentiation and carcinogenesis.3 The kinase activity of c-Src is known to be negatively regulated by phosphorylation of its C-terminal tyrosine 527 residue, whose surrounding sequence is well conserved in c-Yes. Given the high homology between these two kinases and their widely overlapping tissue distributions, it is of little surprise that they are capable of performing redundant functions. c-Src and c-Yes are both activated downstream of a multitude of cell surface receptors, including receptor tyrosine kinases, G-protein-coupled receptors, and cytokine receptors. Additionally, both kinases are activated during the cell cycle transition from G2 to M phase. Despite the evidence for functional overlap, several studies have also indicated specificity between c-Src and c-Yes. The two kinases differ in their sub-cellular localization, intermolecular binding partners, activation in response to cellular stimulation, and ability to mediate downstream signaling.4
REFERENCES
1. Homsi, J. et al: Expert Opin. Ther. Targets 11:91-100, 2007
2. Brickell, P. M. et al: Crit. Rev. Oncol. 3:401-11, 1993 3. Krueger, J. et al: Oncogene 6:933-40, 1991
4. Summy, J.M. et al: J. Cell Sci. 116:2585-98, 2003
2. Brickell, P. M. et al: Crit. Rev. Oncol. 3:401-11, 1993 3. Krueger, J. et al: Oncogene 6:933-40, 1991
4. Summy, J.M. et al: J. Cell Sci. 116:2585-98, 2003
Products are for research use only. They are not intended for human, animal, or diagnostic applications.
Details
Cat.No.: | CP10248 |
Antigen: | Purified recombinant human Yes fragments expressed in E. coli. |
Isotype: | Mouse IgG1 |
Species & predicted species cross- reactivity ( ): | Human |
Applications & Suggested starting dilutions:* | WB 1:1000 IP 1:50 IHC 1:50 ICC n/d FACS n/d |
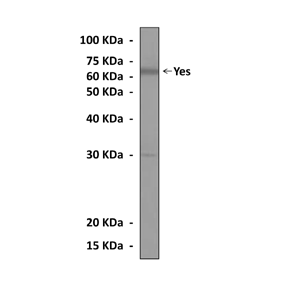
Predicted Molecular Weight of protein: | 62 kDa |
Specificity/Sensitivity: | Detects endogenous Yes proteins without cross-reactivity with other family members. |
Storage: | Store at -20°C, 4°C for frequent use. Avoid repeated freeze-thaw cycles. |
*Optimal working dilutions must be determined by end user.
Products
| Product | Size | CAT.# | Price | Quantity |
|---|---|---|---|---|
| Mouse Yes Antibody: Mouse Yes Antibody | Size: 100 ul | CAT.#: CP10248 | Price: $472.00 |