MSDS Cryopreserved Cells
Instructions HCAEC Normal
5 Important Cell Culture Rules
Cell Apps Flyer Cardiovascular Cells
Cell Apps Flyer Endothelial Cells
Cell Apps Poster Primary Cells
Cell Applications Inc Brochure
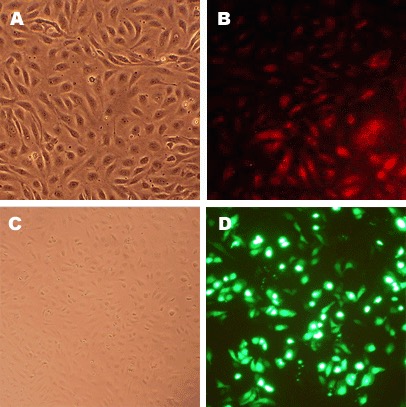
Description
Human Coronary Artery Endothelial Cells (HCAEC) from Cell Applications, Inc. provide an excellent model system to study all aspects of cardiovascular function and disease, and they have been utilized in dozens of research publications, for example to:
- Understand the mechanism of the anti-inflammatory properties of HDL, and demonstrate for the first time that mature miRNA can control gene expression in a cell where it is neither transcribed nor processed
- Study mechanisms of angiogenesis, as well as oxidative stress and inflammation related pathways in endothelia, including gender and race specific differences in patients with peripheral artery disease
- Elucidate molecular mechanisms of various cardiovascular risk factors, including those associated with diabetes
- Understand the mode of action and cardiovascular protection effects of various natural compounds, vitamins and drug candidates
- Develop and evaluate scaffolds and hydrogels for cardiac tissue engineering, and new treatment strategies to prevent stent restenosis
- Compare effects of BMP-4 on HCAEC and Human Pulmonary Artery Endothelial Cells (HPAEC, also from Cell Applications, Inc.)
- Show that only in HCAEC BMP-4 treatment induced ROS, activated NF-kB, ICAM-1 and increased monocyte adhesiveness, explaining why its upregulation leads to atherosclerosis and hypertension in the systemic, but not pulmonary circulation
Additionally, HCAEC, along with human aortic (HAOEC), carotid artery (HCtAEC), subclavian artery (HScAEC) and brachiocephalic artery (HBcAEC), all provided by Cell Applications, Inc., have been used to demonstrate that not only blood vessels from different tissues are highly heterogeneous, they also interact differently with leukocytes during the inflammation response. The authors further showed that differential N-glycosylation of commonly expressed vascular adhesion molecules may be responsible for this heterogeneity, as well as for modulation of signaling under resting and activated inflammatory conditions. This also explains why specific vascular beds may be more or less susceptible to particular diseases or stimuli. Importantly, if cells from different sources were used, these results could not be convincingly validated due to a number of uncontrolled variables, such as age, race, genetic variability or life style choices of the donors. To eliminate the donor-to-donor variability, the scientists took advantage of the great variety of primary cells offered by Cell Applications, including the option of ordering a panel of endothelial cells obtained from different vascular beds of the same donor!
Because of the complex heterogeneity that exists not only between different donors, but even between different vascular beds in the same individual, it would be prudent to confirm any new findings on primary cell lots coming from several different origins.
Details
Tissue | Normal healthy human coronary artery |
QC | No bacteria, yeast, fungi, mycoplasma, virus |
Character | Factor VIII-related Ag, DiI-Ac-LDL uptake |
Bioassay | Attach, spread, proliferate in Growth Med |
Cryovial | 500,000 HCAEC (2nd passage) frozen in Basal Medium w/ 10% FBS, 10% DMSO |
Kit | Cryovial frozen HCAEC (300-05a), Growth Med (212-500), Subculture Rgnt Kit (090K) |
Proliferating | Shipped in Gr Med, 3rd psg (flasks or plates) |
Doublings | At least 15 |
Applications | Laboratory research use only (RUO). Not for human, clinical, diagnostic or veterinary use. |
Products
| Product | Size | CAT.# | Price | Quantity |
|---|---|---|---|---|
| Cryopreserved Human Coronary Artery Endothelial Cells Total Kit, adult: 5x10^5 Cells (Adult), Medium & Subculture Reagents (See Details tab for specifics) | Size: 1 Kit | CAT.#: 300K-05a | Price: $1,094.00 | |
| Cryopreserved Coronary Artery Endothelial Cells (HCAEC), adult: Frozen HCAEC (5x10^5) | Size: 1 Cryovial | CAT.#: 300-05a | Price: $890.00 | |
| Proliferating Coronary Artery Endothelial Cells (HCAEC), adult: Actively growing, dividing cells in medium | Size: T-25 Flask | CAT.#: 301-25a | Price: $890.00 | |
| Proliferating Coronary Artery Endothelial Cells (HCAEC), adult: Actively growing, dividing cells in medium | Size: T-75 Flask | CAT.#: 301-75a | Price: $1,080.00 | |
| Proliferating Coronary Artery Endothelial Cells (HCAEC), adult: Actively growing, dividing cells in medium | Size: 24 Well | CAT.#: 301-24Wa | Price: $1,080.00 | |
| Proliferating Coronary Artery Endothelial Cells (HCAEC), adult: Actively growing, dividing cells in medium | Size: 96 Well | CAT.#: 301-96Wa | Price: $1,200.00 | |
| Cryopreserved Human Coronary Artery Endothelial Cells with plaque Total Kit, adult: 5x10^5 Cells (Plaque, Adult), Medium & Subculture Reagents (See Details tab for specifics) | Size: 1 Kit | CAT.#: 300qK-05a | Price: $1,228.00 | |
| Cryopreserved Coronary Artery Endothelial Cells (HCAEC), Plaque, adult: Frozen HCAEC, Plaque (5x10^5) | Size: 1 Cryovial | CAT.#: 300q-05a | Price: $1,024.00 | |
| Cryopreserved Coronary Artery Endothelial Cells (HCAEC)-AS, adult: Frozen HCAEC-AS from donor with Asthma (5x10^5) | Size: 1 Cryovial | CAT.#: 300AS-05a | Price: $1,024.00 | |
| Cryopreserved Human Coronary Artery Endothelial Cells, with Asthma Total Kit, adult: 5x10^5 Cells (from donor with Asthma, Adult), Medium & Subculture Reagents (See Details tab for specifics) | Size: 1 Kit | CAT.#: 300ASK-05a | Price: $1,228.00 | |
| Cryopreserved Coronary Artery Endothelial Cells, Type 2 Diabetes (HCAEC-T2D), adult: Frozen HCAEC from donor with Type 2 Diabetes (5x10^5) | Size: 1 Cryovial | CAT.#: 300T2D-05a | Price: $1,024.00 | |
| Cryopreserved Human Coronary Artery Endothelial Cells Total Kit, Type 2 Diabetes, adult: 5x10^5 Cells (from donor with Type 2 Diabetes, Adult), Medium & Subculture Reagents (See Details tab for specifics) | Size: 1 Kit | CAT.#: 300T2DK-05a | Price: $1,228.00 |
Related Products
| Product | Size | CAT.# | Price | Quantity |
|---|---|---|---|---|
| Human Meso Endo Growth Medium: All-in-one ready-to-use | Size: 500 ml | CAT.#: 212-500 | Price: $145.00 | |
| Human Meso Endo Growth Medium wo Phenol Red: Growth medium without phenol red | Size: 500 ml | CAT.#: 212PR-500 | Price: $156.00 | |
| Human Meso Endo Growth Medium wo Antibiotics: Growth medium without antibiotics | Size: 500 ml | CAT.#: 212A-500 | Price: $156.00 | |
| Human Meso Endo Growth Medium Kit: Basal medium & growth supplement sold together packaged separately | Size: Yields 500ml | CAT.#: 212K-500 | Price: $156.00 | |
| Human Meso Endo Growth Medium Kit wo Phenol Red: Growth medium kit without phenol red | Size: yields 500 ml | CAT.#: 212KPR-500 | Price: $172.00 | |
| Human Meso Endo Growth Supplement: Added to Basal Medium to create Growth Medium | Size: 30 ml | CAT.#: 212-GS | Price: $81.00 | |
| Human Meso Endo Growth Medium wo FBS: Growth Medium without FBS | Size: 500 ml | CAT.#: 212F-500 | Price: $156.00 | |
| Human Meso Endo Growth Supplement wo FBS: Growth Supplement without FBS | Size: 5 ml | CAT.#: 212F-GS | Price: $91.00 |
Extended Family Products
| Product | Size | CAT.# | Price | Quantity |
|---|---|---|---|---|
| Anti-ICAM-1: Rabbit Intercellular Adhesion Molecule-1 Antibody | Size: 100 ul | CAT.#: CG1238 | Price: $275.00 | |
| Polyclonal Vascular Endothelial Growth Factor Antibody: Polyclonal Vascular Endothelial Growth Factor Antibody | Size: 100 ul | CAT.#: CA1080 | Price: $375.00 | |
| Polyclonal Vascular Endothelial Growth Factor-C Antibody: Polyclonal Vascular Endothelial Growth Factor-C Antibody | Size: 100 ul | CAT.#: CB3778 | Price: $302.00 | |
| Polyclonal VEGF Receptor 1 Antibody: Polyclonal VEGF Receptor 1 Antibody | Size: 100 ul | CAT.#: CB3839 | Price: $333.00 | |
| Freezing Medium: For general cryopreservation of most primary cells. Contains FBS & DMSO. | Size: 50 ml | CAT.#: 040-50 | Price: $54.00 | |
| Cytofect Endothelial Cell Transfection Kit (250 x 24-Wells): 250 x 24-Well Rxns | Size: 1 Kit | CAT.#: TF101K | Price: $546.00 | |
| Cytofect Endothelial Cell Transfection Sample Kit (25 x 24-Wells): 25 x 24-Well Rxns | Size: 1 Sample Kit | CAT.#: TF101KS | Price: $68.00 | |
| Human E-Selectin ELISA Kit: Human E-Selectin ELISA Kit | Size: 96 Wells | CAT.#: CL0501 | Price: $587.00 | |
| Coronary Artery Endothelial Cell RNA (HCAEC RNA), Adult: Total RNA prepared from Human Coronary Artery Endothelial Cells, adult | Size: 10 ug | CAT.#: 300-R10a | Price: $465.00 | |
| Coronary Artery Endothelial Cell RNA (HCAEC RNA), Adult: Total RNA prepared from Human Coronary Artery Endothelial Cells, adult | Size: 25 ug | CAT.#: 300-R25a | Price: $930.00 | |
| Human Heart RNA: Total RNA prepared from human heart tissue | Size: 50 ug | CAT.#: 1H30-50 | Price: $228.00 | |
| Human Heart RNA: Total RNA prepared from human heart tissue | Size: 250 ug | CAT.#: 1H30-250 | Price: $851.00 | |
| Human ICAM-1 ELISA Kit: Human Intercellular Adhesion Molecule-1 ELISA Kit | Size: 96 Wells | CAT.#: CL0370 | Price: $484.00 | |
| Human Gamma-Interferon Inducible Protein 10 (IP-10 / CXCL10): Human gamma-Interferon Inducible Protein 10 | Size: 25 ug | CAT.#: RP1127-25 | Price: $194.00 | |
| Human Gamma-Interferon Inducible Protein 10 (IP-10 / CXCL10): Human gamma-Interferon Inducible Protein 10 | Size: 100 ug | CAT.#: RP1127-100 | Price: $484.00 | |
| Human Gamma-Interferon Inducible Protein 10 (IP-10 / CXCL10): Human gamma-Interferon Inducible Protein 10 | Size: 1000 ug | CAT.#: RP1127-1000 | Price: $3,175.00 | |
| Human P-Selectin ELISA Kit: Human P-Selectin ELISA Kit | Size: 96 wells | CAT.#: CL0505 | Price: $517.00 | |
| Subculture Reagent Kit: 100 ml each of HBSS, Trypsin/EDTA & Trypsin Neutralizing Solution | Size: 1 Kit | CAT.#: 090K | Price: $69.00 | |
| Human Vascular Endothelial Growth Factor-121 (VEGF-121): Human Vascular Endothelial Growth Factor-121 | Size: 10 ug | CAT.#: RP1116-10 | Price: $194.00 | |
| Human Vascular Endothelial Growth Factor-121 (VEGF-121): Human Vascular Endothelial Growth Factor-121 | Size: 100 ug | CAT.#: RP1116-100 | Price: $484.00 | |
| Human Vascular Endothelial Growth Factor-121 (VEGF-121): Human Vascular Endothelial Growth Factor-121 | Size: 1000 ug | CAT.#: RP1116-1000 | Price: $4,090.00 | |
| Human VEGF-c ELISA Kit: Human Vascular Endothelial Growth Factor C ELISA Kit | Size: 96 Wells | CAT.#: CL0588 | Price: $581.00 | |
| Human VEGF-121, Animal-Free: Human Vascular Endothelial Growth Factor-121, Animal-Free | Size: 10 ug | CAT.#: RP1116AF-10 | Price: $213.00 | |
| Human VEGF-121, Animal-Free: Human Vascular Endothelial Growth Factor-121, Animal-Free | Size: 100 ug | CAT.#: RP1116AF-100 | Price: $533.00 | |
| Human VEGF-121, Animal-Free: Human Vascular Endothelial Growth Factor-121, Animal-Free | Size: 1000 ug | CAT.#: RP1116AF-1000 | Price: $4,499.00 |
Resources/Documents
Citations
Publications
2017
Izadifar M, Babyn P, Kelly ME, Chapman D, Chen X. 2017. Bioprinting pattern-dependent electrical/mechanical behavior of cardiac alginate implants: characterization and ex-vivo phase-contrast microtomography assessment. Tissue Eng Part C Methods. doi: 10.1089/ten.TEC.2017.0222.
Clayton, Z., G. Yuenm, S. Sadeghipour, J. Hywood, J. Wong, N. Huang, M. Ng, J. Cooke and S. Patel. 2017. A comparison of the pro-angiogenic potential of human induced pluripotent stem cell derived endothelial cells and induced endothelial cells in a murine model of peripheral arterial disease. Intl J Cardiol, dx.doi.org/10.1016/j.ijcard.2017.01.125.
Dela Paz, N., B. Belchior and J. Frangos. 2017. Shear stress induces Gαq/11 activation independent of G protein-coupled receptor activation in endothelial cells. Am J Physiol – Cell Physiol, DOI: 10.1152/ajpcell.00148.2016.
2016
Aoki, T., K. Yamamoto, M. Fukuda, Y. Shimogonya, S. Fukuda and S. Narumiya. 2016. Sustained expression of MCP-1 by low wall shear stress loading concomitant with turbulent flow on endothelial cells of intracranial aneurysm. Acta Neuropathologica Comm: Neurosci of Dis, 4:48.
Degendorfer, G., C. Chuang, H. Kawasaki, A. Hammer, E. Malle, F. Yamakura and M. Davies. 2016. Peroxynitrite-mediated oxidation of plasma fibronectin. Free Radical Biol & Med, 97:602-615.
Liu, W., B. Liu, S. Liu, J. Zhang and S. Lin. 2016. Sphingosine-1-phosphate receptor 2 mediates endothelial cells dysfunction by PI3K-Akt pathway under high glucose condition. Eur J Pharmacol, 776:19-25.
Qiao, C. 2016. Transcriptomic Profiling Reveals Novel Shear Stress-sensitive Genes in Human Endothelial Cells. PhD Dissertation, U Michigan.
Qiao, C., F. Meng, I. Jang, H. Jo, Y. Chen and J. Zhang. 2016. Deep transcriptomic profiling reveals the similarity between endothelial cells cultured under static and oscillatory shear stress conditions. Physiol Genomics, 48:660-666.
Qin, W., W. Xie, N. Xia, Z. He and T. Sun. 2016. Silencing of Transient Receptor Potential Channel 4 Alleviates oxLDL-induced Angiogenesis in Human Coronary Artery Endothelial Cells by Inhibition of VEGF and NF-κB. Med Sci Monit, 22:930-936.
Ramírez-Sánchez, I., A. Rodríguez, A. Moreno-Ulloa, G. Ceballos and F. Villarreal. 2016. (-)-Epicatechin-induced recovery of mitochondria from simulated diabetes: Potential role of endothelial nitric oxide synthase. Diabetes & Vasc Dis Res, 13:201-210.
Rnjak-Kovacina, J., F. Tang, J. Whitelock and M. Lord. 2016. Silk biomaterials functionalized with recombinant domain V of human perlecan modulate endothelial cell and platelet interactions for vascular applications. Colloids & Surfaces B: Biointerfaces, 148:130-138.
Xu, S., M. Koroleva, M. Yin and Z. Jin. 2016. Atheroprotective laminar flow inhibits Hippo pathway effector YAP in endothelial cells. Translational Research, 176:18-28.
Zhaocheng, J., L. Jinfeng, Y. Luchang, S. Yequan, L. Feng and W. Kai. 2016. Ginkgolide A inhibits lipopolysaccharide-induced inflammatory response in human coronary artery endothelial cells via downregulation of TLR4-NF-κB signaling through PI3K/Akt pathway. Die Pharmazie, 71:588-591.
2015
Boire, T., M. Gupta, A. Zachman, S. Lee, D. Balikov, K. Kim, L. Bellan, and H. Sung. 2015. Pendant Allyl Crosslinking as a Tunable Shape Memory Actuator for Vascular Applications. Acta Biomaterialia, doi:10.1016/j.actbio.2015.06.004.
Degendorfer, G. C. Chuang, A. Hammer, E. Malle, M. and M. Davies. 2015. Peroxynitrous acid induces structural and functional modifications to basement membranes and its key component, laminin. Free Radical Biol & Med, 89:721-733.
Gardner, A., D. Parker, P. Montgomery, D. Sosnowska, A. Casanegra, Z. Ungvari, A. Csiszar, S. Zhang, J. Wang and W. Sonntag. 2015. Influence of diabetes on ambulation and inflammation in men and women with symptomatic peripheral artery disease. J Clin & Translational Endocrinol, 2:137-143.
McGrath, K., X. Li, L. McRobb and A. Heather. 2015. Inhibitory Effect of a French Maritime Pine Bark Extract-Based Nutritional Supplement on TNF-α-Induced Inflammation and Oxidative Stress in Human Coronary Artery Endothelial Cells. Evid-Based Compl & Alt Med, Article ID 260530.
Sung, H., L. Hofmeister, M. Gupta, S. Crowder, S. Yu, A. Zachman, and D. Jung. 2015. Copolymers and methods of use thereof. Patent US9012596B2.
Wu, B., S. Shrestha, K. Ong, D. Johns, L. Dunn, L. Hou, P. Barter and K. Rye. 2015. Increasing HDL levels by inhibiting cholesteryl ester transfer protein activity in rabbits with hindlimb ischemia is associated with increased angiogenesis. Intl J Cardiol, 199:204-212.
Zhong, Y., C. Cheng, Y. Luo, C. Tian, H. Yang, B. Liu, M. Chen, Y. Chen, and S. Liu. 2015. C-reactive protein stimulates RAGE expression in human coronary artery endothelial cells in vitro via ROS generation and ERK/NF-κB activation. Acta Pharmacologica Sinica, 23 March.
2014
Cakan, Y. 2014. Effects of corticosteroid treatment on high-density lipoprotein distribution and function. U Sydney, PhD Thesis, http://hdl.handle.net/2123/12153.
dela Paz, N., B. Melchior, F. Shayo, and J. Frangos. 2014. Heparan sulfates mediate the interaction between platelet endothelial cell adhesion molecule-1 (PECAM-1) and the Gαq/11 subunits of heterotrimeric G proteins. J Biol Chem, 289:7413-7424.
Dela Paz, N., B. Melchior, F. Shayo, and J. Frangos. 2014. Heparan Sulfates Mediate the Interaction Between PECAM-1 and the Gαq/11 Subunits of Heterotrimeric G Proteins. JBC, 289:7413-7424.
Gardner, A.W., D.E. Parker, P.S. Montgomery, D. Sosnowska, A.I. Casanegra, Z. Ungvari, A. Csiszar, and W.E. Sonntag. 2014. Gender and racial differences in endothelial oxidative stress and inflammation in patients with symptomatic peripheral artery disease. Journal of Vascular Surgery: 10.1016/j.jvs.2014.02.045.
Jihan, T., K. Jair, S.R. Aldwin, K.W. Paul, and J.D. Michael. 2014. The smoking-associated oxidant hypothiocyanous acid induces endothelial nitric oxide synthase dysfunction. Biochemical Journal. 457:89-97.
Johnson, T., J. DeQuach, R. Gaetani, J. Ungerleider, D. Elhag,V. Nigam, A. Behfard, and K. Christman. 2014. Human versus porcine tissue sourcing for an injectable myocardial matrix hydrogel. Biomater. Sci. 2:735-744.
Li, H., J. Wang, Y. Wu, L. Zhang, Z. Liu, J. Filep, L. Potempa, Y. Wu and S. Ji. 2014. Topological Localization of Monomeric C-reactive Protein Determines Proinflammatory Endothelial Cell Responses. J Biol Chem, 289:14283-14290.
Liu, S., Y. Zhong, X. You, W. Liu, A. Qun, and S. Ming. 2014.Insulin-like growth factor 1 opposes the effects of C-reactive protein on endothelial cell activation. Molecular and Cellular Biochemistry, 385:199-205.
Lord, M., C. Chuang, J. Melrose, M. Davies, R. Iozzo and J. Whitelock. 2014. The role of vascular-derived perlecan in modulating cell adhesion, proliferation and growth factor signaling. Matrix Biol, 35:112-122.
Lord, M., M. Jung, B. Cheng and J. Whitelock. 2014. Transcriptional complexity of the HSPG2 gene in the human mast cell line, HMC-1. Matrix Biol, 35:123-131.
Morgan, P., P. Sheahan and M. Davies. 2014. Perturbation of Human Coronary Artery Endothelial Cell Redox State and NADPH Generation by Methylglyoxal. PLoS ONE, dx.doi.org/10.1371/journal.pone.0086564.
Tabet, F., K. Vickers, L. Torres, C. Wiese, B. Shoucri, G. Lambert, C. Catherinet, L. Prado-Lourenco, M. Levin, S. Thacker, P. Sethupathy, P. Barter, A. Remaley, and K. Rye. 2014. HDL-transferred microRNA-223 regulates ICAM-1 expression in endothelial cells. Nature Communications 5, Article number 3292.
Tan, J., H. Prosser, L. Vanags, S. Monger, M. Ng and C. Bursill. 2014. High-density lipoproteins augment hypoxia-induced angiogenesis via regulation of post-translational modulation of hypoxia-inducible factor 1α. FASEB J, 28:206-217.
Torella, D., G.M. Ellison, M. Torella, C. Vicinanza, I. Aquila, C. Iaconetti, M. Scalise, F. Marino, B.J. Henning, F.C. Lewis, C. Gareri, N. Lascar, G. Cuda, T. Salvatore, G. Nappi, C. Indolfi, R. Torella, D. Cozzolino, and F.C. Sasso. 2014. Carbonic Anhydrase Activation Is Associated With Worsened Pathological Remodeling in Human Ischemic Diabetic Cardiomyopathy. Journal of the American Heart Association. 3:10.1161/jaha.1113.000434.
Wang, X., A. Zachman, Y. Chun, F. Shen, Y. Hwang and H. Sung. 2014. Polymeric stent materials dysregulate macrophage and endothelial cell functions: Implications for coronary artery stent. Intl J Cardiol, 174:688-695.
2013
Baotic, I., Z.D. Ge, F. Sedlic, A. Coon, D. Weihrauch, D.C. Warltier, and J.R. Kersten. 2013. Apolipoprotein A-1 mimetic D-4F enhances isoflurane-induced eNOS signaling and cardioprotection during acute hyperglycemia. Am J Phys Heart Circ. 305:H219-227.
Candelario, J., and M. Chachisvilis. 2013. Activity of Bradykinin B2 Receptor Is Regulated by Long-Chain Polyunsaturated Fatty Acids. PloS one. 8:e68151.
Castanares-Zapatero, D., C. Bouleti, C. Sommereyns, B. Gerber, C. Lecut, T. Mathivet, M. Horckmans, D. Communi, M. Foretz, J.L. Vanoverschelde, S. Germain, L. Bertrand, P.F. Laterre, C. Oury, B. Viollet, S. Horman, and C. Beauloye. 2013. Connection between cardiac Vascular Permeability, Myocardial Edema, and Inflammation during Sepsis: Role of the alpha1AMP-Activated Protein Kinase Isoform. Crit Care Med. 10.1097/CCM.0b013e31829866dc
Cho, Y.-E., A. Basu, A. Dai, M. Heldak, and A. Makino. 2013. Coronary endothelial dysfunction and mitochondrial reactive oxygen species in type 2 diabetic mice. Am J Physiol Cell Physiol. 305:C1033-1040.
Csiszar, A., D. Sosnowska, Z. Tucsek, T. Gautam, P. Toth, G. Losonczy, R.J. Colman, R. Weindruch, R.M. Anderson, W.E. Sonntag, and Z. Ungvari. 2013. Circulating factors induced by caloric restriction in the nonhuman primate Macaca mulatta activate angiogenic processes in endothelial cells. The journals of gerontology. Series A. 68:235-249.
dela Paz, N.G., B. Melchior, and J.A. Frangos. 2013. Early VEGFR2 activation in response to flow is VEGF-dependent and mediated by MMP activity. Biochemical and biophysical research communications. 434:641-646.
Dunn, L.L., P.J. Simpson, H.G. Prosser, L. Lecce, G.S. Yuen, A. Buckle, D.P. Sieveking, L.Z. Vanags, P.R. Lim, R.W. Chow, Y.T. Lam, Z. Clayton, S. Bao, M.J. Davies, N. Stadler, D.S. Celermajer, R. Stocker, C.A. Bursill, J.P. Cooke, and M.K. Ng. 2013. A Critical Role for Thioredoxin Interacting Protein in Diabetes-Related Impairment of Angiogenesis. Diabetes:doi: 10.2337/db2313-0417
Eppihimer, M.J., N. Sushkova, J.L. Grimsby, N. Efimova, W. Kai, S. Larson, B. Forsyth, B.A. Huibregtse, K.D. Dawkins, and G.J. Wilson. 2013. Impact of Stent Surface on Thrombogenicity and Vascular Healing A Comparative Analysis of Metallic and Polymeric Surfaces. Circulation: Cardiovascular Interventions. 6:370-377.
Gardner, A.W., D.E. Parker, P.S. Montgomery, D. Sosnowska, A.I. Casanegra, O.L. Esponda, Z. Ungvari, A. Csiszar, and W.E. Sonntag. 2013. Impaired Vascular Endothelial Growth Factor A and Inflammation in Patients with Peripheral Artery Disease. Angiology: 0003319713501376.
Hankins, J.L., K.E. Ward, S.S. Linton, B.M. Barth, R.V. Stahelin, T.E. Fox, and M. Kester. 2013. Ceramide-1-phosphate mediates endothelial cell invasion via the annexin a2/p11 heterotetrameric protein complex. J. Biol. Chemistry. 288:19726-19738.
Hiob, M.A., S.G. Wise, A. Kondyurin, A. Waterhouse, M.M. Bilek, M.K.C. Ng, and A.S. Weiss. 2013. The use of plasma-activated covalent attachment of early domains of tropoelastin to enhance vascular compatibility of surfaces. Biomaterials. 34:7584-7591.
Lee, C.-M., J.-A. Gu, T.-G. Rau, C.-H. Yang, W.-C. Yang, S.-H. Huang, F.-Y. Lin, C.-M. Lin, and S.-T. Huang. 2013. Low-Cytotoxic Synthetic Bromorutaecarpine Exhibits Anti-Inflammation and Activation of Transient Receptor Potential Vanilloid Type 1 Activities. BioMed Research International. 2013: Article ID 795095.
Leucker, T.M., Z.-D. Ge, J. Procknow, Y. Liu, Y. Shi, M. Bienengraeber, D.C. Warltier, and J.R. Kersten. 2013. Impairment of Endothelial-Myocardial Interaction Increases the Susceptibility of Cardiomyocytes to Ischemia/Reperfusion Injury. PloS one. 8:e70088.
Lin, L.Y., I.J. Liu, H.C. Chuang, H.Y. Lin, and K.J. Chuang. 2013. Size and composition effects of household particles on inflammation and endothelial dysfunction of human coronary artery endothelial cells. Atmospheric Environment. 77:490-495.
Liu, S.-J., W.-H. Liu, Y. Zhong, and S.-M. Liu. 2013. Glycogen synthase kinase-3β is involved in C-reactive protein-induced endothelial cell activation. Biochemistry (Moscow). 78:915-919.
Lloyd, M.M., M.A. Grima, B.S. Rayner, K.A. Hadfield, M.J. Davies, and C.L. Hawkins. 2013. Comparative reactivity of the myeloperoxidase-derived oxidants hypochlorous acid and hypothiocyanous acid with human coronary artery endothelial cells. Free Radical Biology and Medicine. 65:1352-1362.
Lord, M.S., M. Jung, B. Cheng, and J.M. Whitelock. 2013. Transcriptional complexity of the HSPG2 gene in the human mast cell line, HMC-1. Matrix Biology. 35:123-31
Lord, M.S., B. Tsoi, C. Gunawan, W.Y. Teoh, R. Amal, and J.M. Whitelock. 2013. Anti-angiogenic activity of heparin functionalised cerium oxide nanoparticles. Biomaterials. 34:8808-8818.
Nsimba, M.M., C. Yamamoto, J.N. Lami, Y. Hayakawa, and T. Kaji. 2013. Effect of a Congolese herbal medicine used in sickle cell anemia on the expression of plasminogen activators in human coronary aortic endothelial cells culture. Journal of ethnopharmacology. 146:594-599.
Scott, D.W., M.O. Vallejo, and R.P. Patel. 2013. Heterogenic endothelial responses to inflammation: role for differential N-glycosylation and vascular bed of origin. Journal of the American Heart Association. 2:e000263-e000263.
Takai, J., A. Santu, H. Zheng, S.D. Koh, M. Ohta, L.M. Filimban, V. Lemaître, R. Teraoka, H. Jo, and H. Miura. 2013. Laminar shear stress upregulates endothelial Ca2+-activated K+ channels KCa2.3 and KCa3.1 via a Ca2+/calmodulin-dependent protein kinase kinase/Akt/p300 cascade. American J. of Physiology -Heart and Circulatory Physiology. 305:H484-H493.
Tan, J.T.M., H.C.G. Prosser, L.Z. Vanags, S.A. Monger, M.K.C. Ng, and C.A. Bursill. 2013. High-density lipoproteins augment hypoxia-induced angiogenesis via regulation of post-translational modulation of hypoxia-inducible factor 1α. The FASEB Journal. article fj.13-233874.
2012
Archacki, S.R., G. Angheloiu, C.S. Moravec, H. Liu, E.J. Topol, and Q.K. Wang. 2012. Comparative gene expression analysis between coronary arteries and internal mammary arteries identifies a role for the TES gene in endothelial cell functions relevant to coronary artery disease. Human molecular genetics. 21:1364-1373.
Bailey-Downs, L.C., M. Mitschelen, D. Sosnowska, P. Toth, J.T. Pinto, P. Ballabh, M.N. Valcarcel-Ares, J. Farley, A. Koller, J.C. Henthorn, C. Bass, W.E. Sonntag, Z. Ungvari, and A. Csiszar. 2012. Liver-Specific Knockdown of IGF-1 Decreases Vascular Oxidative Stress Resistance by Impairing the Nrf2-Dependent Antioxidant Response: A Novel Model of Vascular Aging. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 67A:313-329.
Crowder, S.W., M.K. Gupta, L.H. Hofmeister, A.L. Zachman, and H.-J. Sung. 2012. Modular polymer design to regulate phenotype and oxidative response of human coronary artery cells for potential stent coating applications. Acta Biomaterialia. 8:559-569.DeQuach, J. 2012. Decellularized biomaterials for cell culture and repair after ischemic injury. PhD Dissertation, UC San Diego.
Hankins, J. 2012. Re-branding ceramide-1-phosphate: Not just a ceramide metabolite. PhD Dissertation, Penn State U.
Jemy, J. 2012. Does Human Leukocyte Antigen-G (HLA-G) Play a Role in Immunte Modulation and Vasculopathy in Heart Transplantation? Masters Thesis, U Toronto.
Kapur, N.K., C. Shenoy, A.A. Yunis, N.N. Mohammad, S. Wilson, V. Paruchuri, E.E. Mackey, X. Qiao, A. Shah, M.L. Esposito, R.H. Karas, and I.Z. Jaffe. 2012. Distinct Effects of Unfractionated Heparin versus Bivalirudin on Circulating Angiogenic Peptides. PloS one. 7:e34344.
Lin, L.-Y., H.-Y. Lin, H.-W. Chen, T.-L. Su, L.-C. Huang, and K.-J. Chuang. 2012. Effects of temple particles on inflammation and endothelial cell response. Science of The Total Environment. 414:68-72.
Melchior, B., and J.A. Frangos. 2012. Gαq/11-mediated intracellular calcium responses to retrograde flow in endothelial cells. American Journal of Physiology-Cell Physiology. 303:C467-C473.
Ramirez-Sanchez, I., H. Aguilar, G. Ceballos, and F. Villarreal. 2012. (-)-Epicatechin-induced calcium independent eNOS activation: roles of HSP90 and AKT. Molecular and cellular biochemistry. 370:141-150.
Riegel, A. 2012. Pro-inflammatory role of P2Y6 receptor signalling during vascular inflammation. PhD Dissertation, Eberhard Karls Universitat Tubingen.
Tso, C., K.-A. Rye, and P. Barter. 2012. Phenotypic and Functional Changes in Blood Monocytes Following Adherence to Endothelium. PloS one. 7:e37091.
Valcarcel-Ares, M., T. Gautam, J. Warrington, L. Bailey-Down, D. Sosnowska, R. de Cabo, G. Losonczy, W. Sonntag, Z. Ungvari, and A. Csiszar. 2012. Disruption of Nrf2 Signaling Impairs Angiogenic Capacity of Endothelial Cells: Implications for Microvascular Aging. J Gerontol A Biol Sci Med Sci, 67:821-829.
Wu, B.J., K. Chen, P.J. Barter, and K.-A. Rye. 2012. Niacin Inhibits Vascular Inflammation via the Induction of Heme Oxygenase-1. Circulation. 125:150-158.
2011
Aoki, T., M. Nishimura, T. Matsuoka, K. Yamamoto, T. Furuyashiki, H. Kataoka, S. Kitaoka, R. Ishibashi, A. Ishibazawa, and S. Miyamoto. 2011. PGE2‐EP2 signalling in endothelium is activated by haemodynamic stress and induces cerebral aneurysm through an amplifying loop via NF‐κB. British journal of pharmacology. 163:1237-1249.
Archacki, S. 2011. MOLECULAR IDENTIFICATION OF NOVEL GENES ASSOCIATED WITH ATHEROSCLEROSIS. PhD Dissertation, Cleveland State University.
Bowden, J.A., C.J. Albert, O.S. Barnaby, and D.A. Ford. 2011. Analysis of cholesteryl esters and diacylglycerols using lithiated adducts and electrospray ionization-tandem mass spectrometry. Analytical biochemistry. 417:202-210.
Crowder, S.W. 2011. Modular Design of Stent Polymers Regulates Human Coronary Artery Cell Type-Specific Oxidative Response and Phenotype. Vanderbilt University, MSc dissertation.
Di Bartolo, B., L. Vanags, J. Tan, S. Bao, K.-A. Rye, P. Barter, and C. Bursill. 2011. The apolipoprotein A-I mimetic peptide, ETC-642, reduces chronic vascular inflammation in the rabbit. Lipids in health and disease. 10:224.
Kapur, N.K., K.S. Heffernan, A.A. Yunis, T.A. Nguyen, M.J. Aronovitz, P. Parpos, S. Wilson, C.K. Baker, M.L. Esposito, A. Shah, C.D. Kimmelstiel, A. Weintraub, R.H. Karas, and M.E. Mendelsohn. 2011. Elevated Soluble fms-Like Tyrosine Kinase-1 Levels in Acute Coronary Occlusion. Arteriosclerosis, Thrombosis, and Vascular Biology. 31:443-450.
Quinn, K.L., M. Henriques, A. Tabuchi, B. Han, H. Yang, W.-E. Cheng, S. Tole, H. Yu, A. Luo, E. Charbonney, E. Tullis, A. Lazarus, L.A. Robinson, H. Ni, B.R. Peterson, W.M. Kuebler, A.S. Slutsky, and H. Zhang. 2011. Human Neutrophil Peptides Mediate Endothelial-Monocyte Interaction, Foam Cell Formation, and Platelet Activation. Arter., Thromb., & Vasc. Biol. 31:2070-2079.
Singelyn, J.M., and K.L. Christman. 2011. Modulation of material properties of a decellularized myocardial matrix scaffold. Macromolecular bioscience. 11:731-738.
Vladic, N., Z.-D. Ge, T. Leucker, A.K. Brzezinska, J.-H. Du, Y. Shi, D.C. Warltier, P.F. Pratt, and J.R. Kersten. 2011. Decreased tetrahydrobiopterin and disrupted association of Hsp90 with eNOS by hyperglycemia impair myocardial ischemic preconditioning. American Journal of Physiology - Heart and Circulatory Physiology. 301:H2130-H2139.
Wang, M.-Y., S.-R. Ji, C.-J. Bai, D. El Kebir, H.-Y. Li, J.-M. Shi, W. Zhu, S. Costantino, H.-H. Zhou, L.A. Potempa, J. Zhao, J.G. Filep, and Y. Wu. 2011. A redox switch in C-reactive protein modulates activation of endothelial cells. The FASEB Journal. 25:3186-3196.
2010
Bursill, C., M. Castro, D. Beattie, S. Nakhla, E. van der Vorst, A. Heather, P. Barter, and K. Rye. 2010. High-Density Lipoproteins Suppress Chemokines and Chemokine Receptors In Vitro and In Vivo. Arterioscler Thromb Vasc Biol, 30:1773-1778.
Makino, A., T Scott and H. Dillmann. 2010. Mitochondrial fragmentation and superoxide anion production in coronary endothelial cells from a mouse model of type 1 diabetes. Diabetologia, 8:1783-1794.
Hung, C.-H., D. Wu, F.-Y. Lin, R.-Y. Yuan, and C.-J. Hu. 2010. Toll-like Receptor 4 and Vascular Cell Adhesion Molecule 1 in Monocyte-Endothelium Adhesion Induced by Lipopolysaccharide. J. Experimental & Clinical Medicine. 2:297-301.
O'Brien, B.J., J.S. Stinson, S.R. Larsen, M.J. Eppihimer, and W.M. Carroll. 2010. A platinum–chromium steel for cardiovascular stents. Biomaterials. 31:3755-3761.
Rajesh, M., P. Mukhopadhyay, G. Haskó, L. Liaudet, K. Mackie, and P. Pacher. 2010. Cannabinoid-1 receptor activation induces reactive oxygen species-dependent and -independent mitogen-activated protein kinase activation and cell death in human coronary artery endothelial cells. British journal of pharmacology. 160:688-700.
Ramirez-Sanchez, I., L. Maya, G. Ceballos, and F. Villarreal. 2010. (−)-Epicatechin Activation of Endothelial Cell Endothelial Nitric Oxide Synthase, Nitric Oxide, and Related Signaling Pathways. Hypertension. 55:1398-1405.
Seif-Naraghi, S.B., M.A. Salvatore, P.J. Schup-Magoffin, D.P. Hu, and K.L. Christman. 2010. Design and characterization of an injectable pericardial matrix gel: a potentially autologous scaffold for cardiac tissue engineering. Tissue Engin. Part A. 16:2017-2027.
Villareal, F., P. Taub, A. Maisel, G. Schreiner, A. Murphy, K. Yamazaki, and G. Ceballos. 2010. Methods and compositions for treatment of ischemic conditions and conditions related to mitochondrial function. Patent Application US 20120095063 A1.
2009
Anastasiadis, P., and J.S. Allen. 2009. Ultrasound-mediated endothelial cell permeability changes with targeted contrast agents. In Ultrasonics Symposium (IUS), 2009 IEEE International. 16-18.
Csiszar, A., N. Labinskyy, R. Jimenez, J.T. Pinto, P. Ballabh, G. Losonczy, K.J. Pearson, R. de Cabo, and Z. Ungvari. 2009. Anti-oxidative and anti-inflammatory vasoprotective effects of caloric restriction in aging: Role of circulating factors and SIRT1. Mechanisms of Ageing and Development. 130:518-527.
Ji, S.-R., L. Ma, C.-J. Bai, J.-M. Shi, H.-Y. Li, L.A. Potempa, J.G. Filep, J. Zhao, and Y. Wu. 2009. Monomeric C-reactive protein activates endothelial cells via interaction with lipid raft microdomains. The FASEB Journal. 23:1806-1816.
O’Neill, S.M. 2009. Insights into human neutral ceramidase transcription and the role of ceramide metabolism in the development of an inhibitor of restenosis. The Pennsylvania State University, PhD dissertation.
Singelyn, J.M., J.A. DeQuach, S.B. Seif-Naraghi, R.B. Littlefield, P.J. Schup-Magoffin, and K.L. Christman. 2009. Naturally derived myocardial matrix as an injectable scaffold for cardiac tissue engineering. Biomaterials. 30:5409-5416.
2008
Benco, J. 2008. Methods to improve the stability of celluar adhesive proteins and peptides. Patent Application US 20090018642 A1.
Csiszar, A., N. Labinskyy, H. Jo, P. Ballabh, and Z. Ungvari. 2008. Differential proinflammatory and prooxidant effects of bone morphogenetic protein-4 in coronary and pulmonary arterial endothelial cells. Am J Phys. Heart Circ Physiol. 295:H569-577.
O’Brien, B., J. Stinson, D. Boismier, and W. Carroll. 2008. Characterization of an NbTaWZr alloy designed for magnetic resonance angiography compatible stents. Biomaterials, 29:4540-4545.
O’Neil, S., D. Olympia, T. Fox, J. Brown, T. Stover, K. Houck, R. Wilson, P. Waybill, M. Kozak, S. Levison, N. Weber, L. Karavodin, and M. Kester. 2008. C6-Ceramide-Coated Catheters Promote Re-Endothelialization of Stretch-Injured Arteries. Vasc Dis Prev, 5:200-210.
Sieveking, D., A. Buckle, D., Celermajer, and M. Ng. 2008. Strikingly Different Angiogenic Properties of Endothelial Progenitor Cell Subpopulations: Insights From a Novel Human Angiogenesis Assay. J Am Coll Cardiol, 51:660-668.
2007
Csiszar, A., N. Labinskyy, K.E. Smith, A. Rivera, E.N.T.P. Bakker, H. Jo, J. Gardner, Z. Orosz, and Z. Ungvari. 2007. Downregulation of Bone Morphogenetic Protein 4 Expression in Coronary Arterial Endothelial Cells: Role of Shear Stress and the cAMP/Protein Kinase A Pathway. Arteriosclerosis, Thrombosis, and Vascular Biology. 27:776-782.
Mukhopadhyay, P., M. Rajesh, G. Hasko, B.J. Hawkins, M. Madesh, and P. Pacher. 2007. Simultaneous detection of apoptosis and mitochondrial superoxide production in live cells by flow cytometry and confocal microscopy. Nat. Protocols. 2:2295-2301.
Rajesh, M., P. Mukhopadhyay, S. Bátkai, G. Haskó, L. Liaudet, J.W. Huffman, A. Csiszar, Z. Ungvari, K. Mackie, and S. Chatterjee. 2007. CB2-receptor stimulation attenuates TNF-α-induced human endothelial cell activation, transendothelial migration of monocytes, and monocyte-endothelial adhesion. American journal of physiology. Heart and circulatory physiology. 293:H2210.
2006
Wildsmith, K., C. Albert, D. Anbukumar, and D. Ford. 2006. Metabolism of Myeloperoxidase-derived 2-Chlorohexadecanal. J Biol Chem, 281:16849-16860.
2004
Sharifi, B., and P. Shah. 2004. Use of pleiotrophin in the diagnosis, treatment and prevention of disease. Patent Application US 20070065409 A1.
2003
Liu, Y.-C., Y.-C. Lo, C.-W. Huang, and S.-N. Wu. 2003. Inhibitory action of ICI-182,780, an estrogen receptor antagonist, on BK Ca channel activity in cultured endothelial cells of human coronary artery. Biochemical pharmacology. 66:2053-2063.
Ng, M., S. Nakhla, A. Baoutina, W. Jessup, D. Handelsman, and D. Celermajer. 2003. Dehydroepiandrosterone, an adrenalandrogen, increases human foam cell formation. J Am Coll Cardio, 42:1967-1974.