Product Sheet CL0667
Description
BACKGROUND Cadherins are a superfamily of glycoproteins that mediate Ca2+-dependent, homotypic cell–cell adhesion in all solid tissues of the organism. They are known to play important roles in maintaining the structural integrity of epithelial tissues and in tissue morphogenesis and to serve as signalling molecules to regulate cell behaviour, especially in some kinds of tumors. The classical cadherins are the best characterized members of the family and are designated by the originally described tissue specificity, including E- (epithelial) (CDH1), N- (neural) (CDH2) and P- (placental)(CDH3) cadherins.1 E-cadherin is the prototype family member of classical cadherins, single-span transmembrane glycoproteins that interact in a calcium-dependent, hemophilic manner with E-cadherins on neighboring cells. E-cadherin-mediated cell-cell adhesion complexes are anchored to the actin cytoskeleton via its cytoplasmic domain, beta-catenin and alpha-catenin. N-cadherin also forms hemophilic cell-cell adhesion junctions. It is normally expressed in nervous tissue, vascular endothelial cells and in skeletal and cardiac muscle cells. It is found to be localized in the lamellipodia and fillopodia.
P-cadherin (CDH3) is a 118-kd transmembrane glycoprotein and first identified in mouse placenta. It is composed of 5 extracellular domains, designated EC1–EC5, a transmembrane domain and a small intracellular domain. The extracellular domains, mainly EC1, are crucial to the normal alignment of the protein in order to form the appropriate adhesive interactions with nearby cells. The EC domains contain calcium-binding regions that are imperative for their normal functioning. The intracellular domain of P-cadherin interacts with beta-catenin, which binds, indirectly through alpha-catenin, to the actin filament-binding proteins and other actin-binding proteins and functions in maintaining the cytoskeleton of the cells. More importantly, beta-catenin is involved in the Wnt signaling pathway, influencing many developmental processes. The function of P-cadherin is not restricted to the formation of adherens junctions but is also involved in other biological processes, such as cell recognition, cell signaling, morphogenesis and tumor development.3 In human its expression is not detectable in the placenta but is present in a few organs, such as mammary gland and prostate. E-Cadherin is expressed in most epithelial cells, whereas P-cadherin is restricted to basal cell layers, including basal cells of skin and prostate and myoepithelial cells of the mammary gland. In addition to maintaining cellular adhesion, P-cadherin plays important role in cell differentiation and proliferation.2 It was shown that P-cadherin expression pattern has been linked to a more dedifferentiated stem-cell-related population of tumor cells. Up-regulation of P-cadherin has been shown in several lesions, including breast cancer, in which there is usually down-regulation of E-cadherin. Breast carcinomas show aberrant P-cadherin expression in ∼30% of the cases and has been reported as a prognostic marker of poor outcome in patients. It was shown that P-cadherin overexpression, in breast cancer cells promotes cell invasion, motility and migration. Moreover, the overexpression of P-cadherin induces the secretion of matrix metalloproteases, specifically MMP-1 and MMP-2, which then lead to P-cadherin ectodomain cleavage. Furthermore, soluble P-cadherin fragment is able to induce in vitro invasion of breast cancer cells.4 Mutations in P-cadherin could result in development hypotrichosis with juvenile macular dystrophy (HJMD).
P-cadherin (CDH3) is a 118-kd transmembrane glycoprotein and first identified in mouse placenta. It is composed of 5 extracellular domains, designated EC1–EC5, a transmembrane domain and a small intracellular domain. The extracellular domains, mainly EC1, are crucial to the normal alignment of the protein in order to form the appropriate adhesive interactions with nearby cells. The EC domains contain calcium-binding regions that are imperative for their normal functioning. The intracellular domain of P-cadherin interacts with beta-catenin, which binds, indirectly through alpha-catenin, to the actin filament-binding proteins and other actin-binding proteins and functions in maintaining the cytoskeleton of the cells. More importantly, beta-catenin is involved in the Wnt signaling pathway, influencing many developmental processes. The function of P-cadherin is not restricted to the formation of adherens junctions but is also involved in other biological processes, such as cell recognition, cell signaling, morphogenesis and tumor development.3 In human its expression is not detectable in the placenta but is present in a few organs, such as mammary gland and prostate. E-Cadherin is expressed in most epithelial cells, whereas P-cadherin is restricted to basal cell layers, including basal cells of skin and prostate and myoepithelial cells of the mammary gland. In addition to maintaining cellular adhesion, P-cadherin plays important role in cell differentiation and proliferation.2 It was shown that P-cadherin expression pattern has been linked to a more dedifferentiated stem-cell-related population of tumor cells. Up-regulation of P-cadherin has been shown in several lesions, including breast cancer, in which there is usually down-regulation of E-cadherin. Breast carcinomas show aberrant P-cadherin expression in ∼30% of the cases and has been reported as a prognostic marker of poor outcome in patients. It was shown that P-cadherin overexpression, in breast cancer cells promotes cell invasion, motility and migration. Moreover, the overexpression of P-cadherin induces the secretion of matrix metalloproteases, specifically MMP-1 and MMP-2, which then lead to P-cadherin ectodomain cleavage. Furthermore, soluble P-cadherin fragment is able to induce in vitro invasion of breast cancer cells.4 Mutations in P-cadherin could result in development hypotrichosis with juvenile macular dystrophy (HJMD).
REFERENCES
1. Hulpiau, P. & van Roy, F.: Int. J. Biochem. Cell Biol. 41: 349–69, 2009
2. Shimomura, Y. et al: Dermatol. 220:208-12, 2010
3. Takeichi, M. et al: Curr. Opin. Cell Biol. 7:619-27, 1995
4. Ribeiro, A.S. et al: Oncogene 29:392-402, 2010
2. Shimomura, Y. et al: Dermatol. 220:208-12, 2010
3. Takeichi, M. et al: Curr. Opin. Cell Biol. 7:619-27, 1995
4. Ribeiro, A.S. et al: Oncogene 29:392-402, 2010
Products are for research use only. They are not intended for human, animal, or diagnostic applications.
Details
Cat.No.: | CL0667 |
Target Protein Species: | Human |
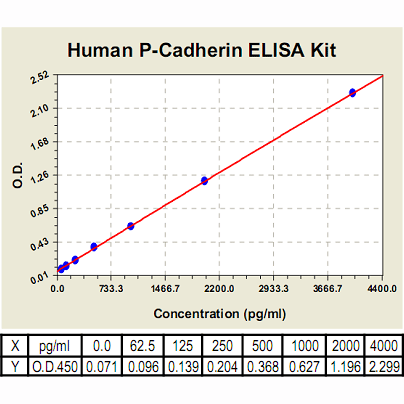
Range: | 62.5pg/ml-4, 000pg/ml |
Specificity: | No detectable cross-reactivity with any other cytokine. |
Storage: | Store at 4°C. Use within 6 months. |
ELISA Kits are based on standard sandwich enzyme-linked immunosorbent assay technology. Freshly prepared standards, samples, and solutions are recommended for best results.
Products
| Product | Size | CAT.# | Price | Quantity |
|---|---|---|---|---|
| Human P-Cadherin ELISA Kit: Human P-Cadherin ELISA Kit | Size: 96 wells | CAT.#: CL0667 | Price: $554.00 |