Product Sheet CL0532
Description
BACKGROUND Tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL) or Apo2 ligand ( Apo2L) is one of several members of the TNF gene superfamily that induce apoptosis through engagement of death receptors. TRAIL/ Apo2L is unusual as compared to any other cytokine as it interacts with a complex system of receptors: two pro-apoptotic death receptors and three anti-apoptotic decoys. This protein has generated tremendous excitement as a potential tumor-specific cancer therapeutic because, as a stable soluble trimer, it selectively induces apoptosis in many transformed cells but not in normal cells. Transcriptional activation of TRAIL/ Apo2L by interferons (IFNs) through specific regulatory elements in its promoter, and possibly by a number of other cytokines, reveals its possible involvement in the activation of natural killer cells, cytotoxic T lymphocytes, and dendritic cells.1
The interaction of TRAIL with its two death receptors DR4 (TRAIL-R1) and DR5 (TRAIL-R2) is the initial step in TRAIL-induced apoptosis. The binding of TRAIL leads to trimerization of the death receptors and activation of receptor-mediated death pathway. The activated death receptors recruit and activate an adaptor protein called Fas-associated death domain (FADD) through interactions between the death domain (DD) on the death receptors and FADD. The death effector domain (DED) of FADD recruits and activates caspase-8, leading to the formation of the death-inducing signaling complex (DISC). In type I cells, the presence of activated caspase-8, is sufficient to induce activation of one or more effector caspases (e.g., caspase-3 or -7), which then act on final death substrates in apoptosis. However, in type II cells, even a small amount of activated caspase-8, although not enough to activate the effector caspases, is sufficient to trigger a mitochondria-dependent apoptotic amplification loop by activating Bid, which induces the accumulation of Bax in mitochondria, the release of cytochrome c from mitochondria, the activation of caspase-9, caspase-3, and caspase-7, and finally, programmed cell death.2 In addition to inducing apoptosis by caspase-8 recruitment through FADD, TRAIL binding to its receptors also leads to activation of the transcription factor nuclear factor-kappa B (NF-kB). TRAIL death receptors, like the TNF receptor 1 (TNFR1), activate NF- NF-kB through the TNFR1-associated death domain protein (TRADD). Activated TRADD recruits the DD-containing protein RIP and TNF receptor-associated factor-2 (TRAF2), leading to activation of the NF-kB pathway; in contrast, dominant-negative TRADD can block the NF-kB activation induced by TRAIL receptors. In TNFR1 signaling, TRADD is believed to be upstream of FADD. However, in TRAIL signaling, TRADD may be mediated by FADD, because TRADD recruitment to the DISC is observed only in the presence of FADD.3
The interaction of TRAIL with its two death receptors DR4 (TRAIL-R1) and DR5 (TRAIL-R2) is the initial step in TRAIL-induced apoptosis. The binding of TRAIL leads to trimerization of the death receptors and activation of receptor-mediated death pathway. The activated death receptors recruit and activate an adaptor protein called Fas-associated death domain (FADD) through interactions between the death domain (DD) on the death receptors and FADD. The death effector domain (DED) of FADD recruits and activates caspase-8, leading to the formation of the death-inducing signaling complex (DISC). In type I cells, the presence of activated caspase-8, is sufficient to induce activation of one or more effector caspases (e.g., caspase-3 or -7), which then act on final death substrates in apoptosis. However, in type II cells, even a small amount of activated caspase-8, although not enough to activate the effector caspases, is sufficient to trigger a mitochondria-dependent apoptotic amplification loop by activating Bid, which induces the accumulation of Bax in mitochondria, the release of cytochrome c from mitochondria, the activation of caspase-9, caspase-3, and caspase-7, and finally, programmed cell death.2 In addition to inducing apoptosis by caspase-8 recruitment through FADD, TRAIL binding to its receptors also leads to activation of the transcription factor nuclear factor-kappa B (NF-kB). TRAIL death receptors, like the TNF receptor 1 (TNFR1), activate NF- NF-kB through the TNFR1-associated death domain protein (TRADD). Activated TRADD recruits the DD-containing protein RIP and TNF receptor-associated factor-2 (TRAF2), leading to activation of the NF-kB pathway; in contrast, dominant-negative TRADD can block the NF-kB activation induced by TRAIL receptors. In TNFR1 signaling, TRADD is believed to be upstream of FADD. However, in TRAIL signaling, TRADD may be mediated by FADD, because TRADD recruitment to the DISC is observed only in the presence of FADD.3
REFERENCES
1. Aggarwal, B.B. et al: Vitam. Horm. 67:453-83, 2004
2. Srivastava, R.K.: Neoplasia 3:535-46, 2001
3. Yang, X. & Thiele, C.J.: Cancer Lett. 197:137-43, 2003
2. Srivastava, R.K.: Neoplasia 3:535-46, 2001
3. Yang, X. & Thiele, C.J.: Cancer Lett. 197:137-43, 2003
Products are for research use only. They are not intended for human, animal, or diagnostic applications.
Details
Cat.No.: | CL0532 |
Target Protein Species: | Human |
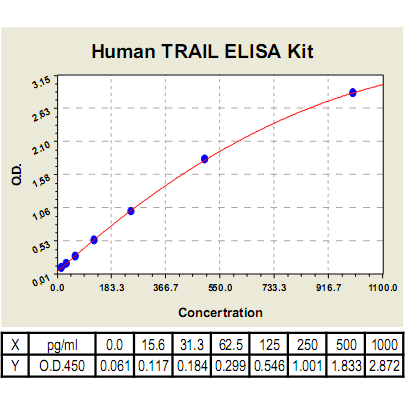
Range: | 15.6pg/ml-1000pg/ml |
Specificity: | No detectable cross-reactivity with any other cytokine. |
Storage: | Store at 4°C. Use within 6 months. |
ELISA Kits are based on standard sandwich enzyme-linked immunosorbent assay technology. Freshly prepared standards, samples, and solutions are recommended for best results.
Products
| Product | Size | CAT.# | Price | Quantity |
|---|---|---|---|---|
| Human TRAIL ELISA Kit: Human Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand ELISA Kit | Size: 96 Wells | CAT.#: CL0532 | Price: $554.00 |